
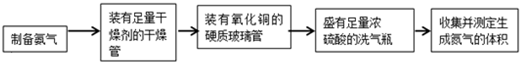
| ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� | |
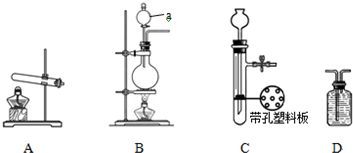
| ��С�� | A | �������ơ������ | ��Ӧ�Ļ�ѧ����ʽΪ �� ��NH4��2SO4+Ca��OH��2�T2NH3��+2H2O+CaSO4 ��NH4��2SO4+Ca��OH��2�T2NH3��+2H2O+CaSO4 |
| ���� | �� B B |
Ũ��ˮ���������� |
| m1-m2 |
| 16 |
| m1-m2 |
| 16 |
| V1��2 |
| 22.4 |
| V1��2 |
| 22.4 |
| m1-m2 |
| 16 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
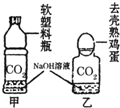 ��2008?������ʵ���Ҽ���CO2ͨ���ó���ʯ��ˮ��������CO2ͨ����NaOH��Һ����CO2��NaOH��Һ��Ӧʱ���������������ѧ�о���ѧϰС������֤��CO2��NaOH��Һȷʵ�����˻�ѧ��Ӧ����̽����
��2008?������ʵ���Ҽ���CO2ͨ���ó���ʯ��ˮ��������CO2ͨ����NaOH��Һ����CO2��NaOH��Һ��Ӧʱ���������������ѧ�о���ѧϰС������֤��CO2��NaOH��Һȷʵ�����˻�ѧ��Ӧ����̽����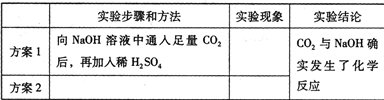
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ڶ�ʮ���조��ԭ����ȫ������ѧ����ѧ���ʺ�ʵ��������������������������Ծ��������棩 ���ͣ������


| ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� | |
| ��С�� | A | �������ơ������ | ��Ӧ�Ļ�ѧ����ʽΪ �� |
| ��С�� | �� | Ũ��ˮ���������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com