
| 100 |
| 44 |
| x |
| 4.4g |
| 2.5g |
| 12.5g |
| 40 |
| 100 |
| 4g |
| 40% |


 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ȼ��һ���ǻ�ѧ�仯 |
| B�������ݳ��ֵı仯�ǻ�ѧ�仯 |
| C��������ȵı仯�������仯 |
| D��״̬�ĸı�һ���������仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
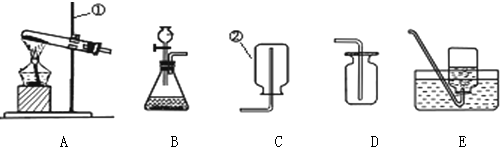
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
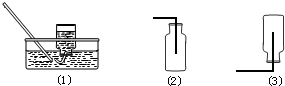
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
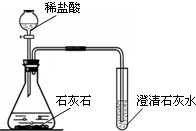 ij��ѧ��ȤС������ͼװ����ȡ�����������̼��ʵ������У��ɹ۲쵽�Թ������
ij��ѧ��ȤС������ͼװ����ȡ�����������̼��ʵ������У��ɹ۲쵽�Թ������| ʵ����� | ʵ������ | ʵ����� |
| ʵ���һСƬpH��ֽ����һ��ɾ��IJ���Ƭ�ϣ��� |
��ñ���Һ��pH=8 | ����� ������������������� |
| ʵ���ȡ�����ܽ�ɵij�����Һ����һ֧�Թ��У����� |
�����ݲ��� | ��Ӧ�Ļ�ѧ����ʽΪ�� ����ڳ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
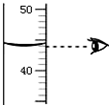 ��Ͳ����;����ȡҺ�����������ʱ����Ͳ�����ƽ������Ҫ����Ͳ��Һ��
��Ͳ����;����ȡҺ�����������ʱ����Ͳ�����ƽ������Ҫ����Ͳ��Һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com