
| 0.8g | ||
|
| 4g |
| 5g |


 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?���ڣ�ij������Ʒ˵����IJ�����Ϣ��ͼ��ʾ��С��Ϊ�˼���ò�Ʒ�Ƿ�ϸ�ȡ10Ƭ����Ʒ����100gϡ�����У���Ч�ɷָպ���ȫ��Ӧ�������ɷֲ������ᷴӦ����ʣ������Һ���������ȷ�Ӧǰ������2.2g��������������⣺
��2012?���ڣ�ij������Ʒ˵����IJ�����Ϣ��ͼ��ʾ��С��Ϊ�˼���ò�Ʒ�Ƿ�ϸ�ȡ10Ƭ����Ʒ����100gϡ�����У���Ч�ɷָպ���ȫ��Ӧ�������ɷֲ������ᷴӦ����ʣ������Һ���������ȷ�Ӧǰ������2.2g��������������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����б�ҵ��ѧ���ԣ��㶫���ھ�����ѧ���������� ���ͣ�������
��4�֣���2012?���ڣ�ij������Ʒ˵����IJ�����Ϣ��ͼ��ʾ��С��Ϊ�˼���ò�Ʒ�Ƿ�ϸ�ȡ10Ƭ����Ʒ����100gϡ�����У���Ч�ɷָպ���ȫ��Ӧ�������ɷֲ������ᷴӦ����ʣ������Һ���������ȷ�Ӧǰ������2.2g��������������⣺
��1������������2.2g������Ϊ��������_________�����壻
��2��10Ƭ��Ƭ��CaCO3������Ϊ��_________��g��
��3��10Ƭ��Ƭ��ʵ�ʺ�����Ϊ��_________��g��
��4���ò���Ʒ��������_________������ϸ��ϸ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����б�ҵ��ѧ���ԣ��㶫���ھ�����ѧ���������� ���ͣ��ƶ���
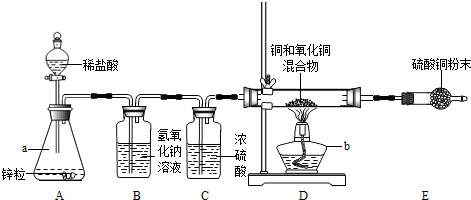
��5�֣���2012?���ڣ�С��Ϊ�˲ⶨͭ������ͭ�����������ͭ�����������������װ����ͼ��ʵ��װ��
��ͼ�ش��������⣺
��1������ʶ��a��_________����b��_________����
��2��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��_________����
��3���ܳ�ȥH2�л�������HCl�����װ������_________������װ����ţ���
��4��װ��E������ͭ��ĩ����ɫ��˵��Ӳ���Թ��з�Ӧ������_________�����ɣ�
��5��ʵ��ǰӲ���Թ��л���������Ϊ5g��ʵ���ʣ���������Ϊ4.2g��������������ͭ������������_________��%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����б�ҵ��ѧ���ԣ��㶫���ھ�����ѧ�������棩 ���ͣ�̽����
��5�֣���2012•���ڣ�С��Ϊ�˲ⶨͭ������ͭ�����������ͭ�����������������װ����ͼ��ʵ��װ��
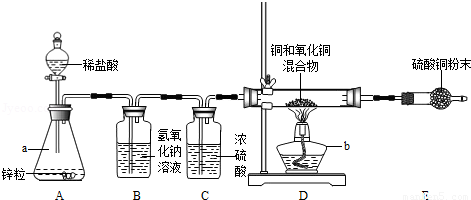
��ͼ�ش��������⣺
��1������ʶ��a��_________����b��_________����
��2��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��_________����
��3���ܳ�ȥH2�л�������HCl�����װ������_________������װ����ţ���
��4��װ��E������ͭ��ĩ����ɫ��˵��Ӳ���Թ��з�Ӧ������_________�����ɣ�
��5��ʵ��ǰӲ���Թ��л���������Ϊ5g��ʵ���ʣ���������Ϊ4.2g��������������ͭ������������_________��%��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com