��2012?������ģ�⣩�ҹ�����ר�Һ�°�ġ������Ƽ����Ϊ�����Ƽҵ������ͻ�����ף�����NaCl��NH
3��CO
2��Ϊԭ�����Ƶ�NaHCO
3����������������йط�Ӧ�Ļ�ѧ����ʽΪ��
NH
3+CO
2+H
2O�TNH
4HCO
3��
NH
4HCO
3+NaCl�TNaHCO
3��+NH
4Cl��
2NaHCO
3Na
2CO
3+CO
2��+H
2O
�ش��������⣺
��1��̼������뱥��ʳ��ˮ��Ӧ��������̼�����ƾ����ԭ����
c
c
������ĸ��ţ���
a��̼������������ˮ b��̼�����������ֽ� c��̼�����Ƶ��ܽ����Խ�С����������Һ�����Ƚᾧ����
��2��ij̽���С����������Ƽ�ԭ��������̼�����Ƶ��Ʊ�ʵ�飬ͬѧ�ǰ�������Ƶķ���ʵ�飮
��һλͬѧ��������̼����ͨ�뺬���ı���ʳ��ˮ���Ʊ�̼�����ƣ�ʵ��װ������ͼ��ʾ��ͼ�мг֡��̶��õ�����δ��������
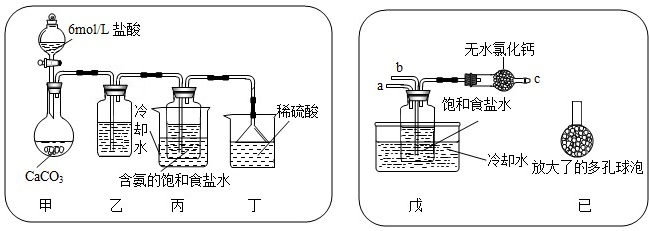
�Իش������й����⣺
������װ���е��Լ��� ���͵�̼��������Һ��������
���ռ�װ�ÿ��ܻӷ������Ȼ�������
���ռ�װ�ÿ��ܻӷ������Ȼ�������
��
����װ����ϡ�����������
����ĩ��Ӧ��NH3
����ĩ��Ӧ��NH3
��
����ʵ����������NaHCO
3����IJ�����
����
����
���������������ƣ���
����һλͬѧ��ͼ����װ�ã�����װ��δ����������ʵ�飮
����ʵ��ʱ�����ȴ�
a
a
��ͨ��
����
����
���壻
������ͬѧ��������װ�õ�b���¶����Ӽ�װ�ã�������
������������Һ�Ӵ���������CO2������
������������Һ�Ӵ���������CO2������
��
��3��������д��һ��ʵ������ȡ����̼�����Ƶķ�����
��̼���������������ʳ��ˮ��Ӧ���������ռ���Һ��ͨ�����CO2��������Na2CO3 ��Һ��ͨ�����CO2 �ȣ����������������ɣ�
��̼���������������ʳ��ˮ��Ӧ���������ռ���Һ��ͨ�����CO2��������Na2CO3 ��Һ��ͨ�����CO2 �ȣ����������������ɣ�
��
��4���������г�������������Ȼ��ƣ���ij�о���ѧϰС����һ�����ֻ���Ǻ��Ȼ��ƣ�Ϊ�о�����̽��������Ʒ��̼���Ƶĺ�����
��ʵ����ơ�
����
�����˼·��������Ʒ���Ȼ�����Һ��Ӧ���ɳ���̼��Ƶ����������̼���Ƶ��������ټ�����Ʒ��̼���Ƶ�����������
�������裺��ȡ13.25g������Ʒ������������Ȼ�����Һ����ֽ��裮���ˡ�ϴ�ӡ�����õ��İ�ɫ����10.00g��
�����ݴ��������������ʵ�����ݣ��������Ʒ��̼���Ƶ�����������
������̣�
�⣺�贿����Ʒ�к�Na
2CO
3������Ϊx
Na
2CO
3+CaCl
2�TCaCO
3��+2NaCl
106 100
x 10.00g
=x=10.6g
������Ʒ��Na
2CO
3����������Ϊ
��100%=80%
�⣺�贿����Ʒ�к�Na
2CO
3������Ϊx
Na
2CO
3+CaCl
2�TCaCO
3��+2NaCl
106 100
x 10.00g
=x=10.6g
������Ʒ��Na
2CO
3����������Ϊ
��100%=80%
�ҷ���
I�����˼·��������Ʒ������Ϊa g����ϡ������ȫ��Ӧ���ɶ�����̼������������Ϊb g�������̼���Ƶ��������ټ�����Ʒ��̼���Ƶ�����������
II��ʵ����ۣ�
��1����a��b��������ϵ����
����д��a��b��ĸ�ı���ʽ����ͬ��ʱ��������Ʒ��ֻ����̼���ƣ���Ʒ��̼���Ƶ�����������100%��
��2����a��b��������ϵ����
ʱ��������Ʒ����̼���ƺ������Ȼ�����ɵĻ�����Ʒ��̼���Ƶ�����������
��
��ʵ�����ۡ�
��������
��һ�������У���ɫ�����������ڹ��ˡ�����Ȳ��������л�������ģ���ɼ�������ʵ��ֵ���ƫС��������Ȼ�����Һ�����Ȼ�����Һ�������ʹ����С��������
BaCl2��CaCl2����Է������������ij��������������С
BaCl2��CaCl2����Է������������ij��������������С
��
�������ҷ����У��в������ɵĶ�����̼�������ܽ���ˮ��û��ȫ���ݳ�����ɼ�������ʵ��ֵ���
ƫС
ƫС
���ƫ����ƫС�������䡱����



 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
