
��һ���������ı�ǩ����ͼ��

��1�����������ƵĻ�ѧʽ��Na2PO3 F������Է�������Ϊ ��
��2����֧���ຬ��������Ϊ g��
��3������ˮ��Һ��ʹpH��ֽ����������� (���"����"�)�ԡ�
��4�����ò����[ ��ѧʽΪ(NH4)2C2O4 ]��������Ħ�����������ӣ����ɲ���Ƴ���������狀��Ȼ�����Һ��Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ�Ļ�������Ϊ ��Ӧ��
��5��ijͬѧ�������ʵ�鷽���ⶨ�����и�Ԫ�ص�������������һ���������м������ϡ���ᣬ�ⶨ����CO2���������ݴ˼��������и�Ԫ�ص�����������С��ͬѧ��Ϊ��ʹ�ų�ʵ�������Ͳ�����Ӱ�죬�����ⶨ�Ľ���Բ�һ��ȷ�������� ��(����ĸ��2��)
A�������п��ܴ�������̼���Σ��ᵼ�½��ƫ�ߣ�
B�������п��ܴ������������ƵĻ�����ᵼ�½��ƫ��
��1��144 ��2��0.28 ��3����
(4)��NH4��2C2O4+CaCl2�TCaC2O4��+2NH4Cl (��ѧʽ����ƽ��ȷ��1��)
���ֽ� (5) AB
��������
�����������1��Na2PO3 F��Է�������Ϊ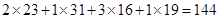 ����2����֧���ຬ��������Ϊ
����2����֧���ຬ��������Ϊ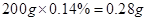 ����3����ʹpH��ֽ����������ʼ��ԣ���4������狀��Ȼ�����Һ��ӦΪ���ֽⷴӦ����ѧ����ʽΪ��NH4��2C2O4+CaCl2�TCaC2O4��+2NH4Cl����5�������п��ܺ�����������Ӱ��ⶨ�����
����3����ʹpH��ֽ����������ʼ��ԣ���4������狀��Ȼ�����Һ��ӦΪ���ֽⷴӦ����ѧ����ʽΪ��NH4��2C2O4+CaCl2�TCaC2O4��+2NH4Cl����5�������п��ܺ�����������Ӱ��ⶨ�����
���㣺��ѧʽ������
��������ѧʽ�����������������п��г��ֽ϶࣬�ѵ����������ϣ�ע��ϸ�Ķ��⣬��Ҫ�뵱Ȼ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����Ҫ���Գɷ֡����������ƣ�Na2PO3F�� ��Ħ������̼��� �����͡���ˬ���� ����������0.14% ����������200g �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ�  ��һ���������ı�ǩ��ͼ�� ��һ���������ı�ǩ��ͼ����1�����������ƵĻ�ѧʽ��Na2PO3 F������Է�������Ϊ 144 144 ��2����֧���ຬ��������Ϊ 0.28 0.28 g����3������ˮ��Һ��ʹpH��ֽ����������� �� �� ����ᡰ���С�����ԣ���4�����ò����[��ѧʽΪ��NH4��2C2O4]��������Ħ�����������ӣ����ɲ���Ƴ���������狀��Ȼ�����Һ��Ӧ�Ļ�ѧ����ʽΪ ��NH4��2C2O4+CaCl2�TCaC2O4��+2NH4Cl ��NH4��2C2O4+CaCl2�TCaC2O4��+2NH4Cl ���÷�Ӧ�Ļ�������Ϊ���ֽ� ���ֽ� ��Ӧ����5��ijͬѧ�������ʵ�鷽���ⶨ�����и�Ԫ�ص�������������һ���������м������ϡ���ᣬ�ⶨ����CO2���������ݴ˼��������и�Ԫ�ص�����������С��ͬѧ��Ϊ��ʹ�ų�ʵ�������Ͳ�����Ӱ�죬�����ⶨ�Ľ���Բ�һ��ȷ�������� AB AB ��������ĸ��A�������п��ܴ�������̼���Σ��ᵼ�½��ƫ�ߣ� B�������п��ܴ������������ƵĻ�����ᵼ�½��ƫ�ͣ� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ�  ��һ���������ı�ǩ��ͼ�� ��һ���������ı�ǩ��ͼ����1�����������ƵĻ�ѧʽ��Na2PO3F������Է�������Ϊ 144 144 ������PԪ�صĻ��ϼ�Ϊ+5����FԪ�صĻ��ϼ�Ϊ-1 -1 ����2����֧���ຬ��������Ϊ 0.28 0.28 g����3������������ֻ�е��������ƺ���������֧�����е��������Ƶ������Ƕ��٣�����ʽ���㣬�������2λС���� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ�  ��һ���������ı�ǩ����ͼ�� ��һ���������ı�ǩ����ͼ����1�����������ƵĻ�ѧʽ��Na2PO3F������PԪ�صĻ��ϼ�Ϊ+5����FԪ�صĻ��ϼ�Ϊ -1 -1 ����2������ˮ��Һ��ʹpH��ֽ����������� �� �� �ԣ���ᡱ�����С��������3������ʦ������ͳ���ʯ��ˮ�����Ħ�����е�������ȷʵ��̼�������ط�Ӧ�Ļ�ѧ����ʽΪ�� CaCO3+2HCl=CaCl2+CO2��+H2O CaCO3+2HCl=CaCl2+CO2��+H2O ����Ca��OH��2+CO2=CaCO3��+H2O Ca��OH��2+CO2=CaCO3��+H2O ���鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ���������ؾ��꼶���£��¿���ѧ�Ծ���3�·ݣ��������棩 ���ͣ������ ��һ���������ı�ǩ����ͼ�� ��1�����������ƵĻ�ѧʽ��Na2PO3F������PԪ�صĻ��ϼ�Ϊ+5����FԪ�صĻ��ϼ�Ϊ �� ��2������ˮ��Һ��ʹpH��ֽ����������� �ԣ���ᡱ�����С������ ��3������ʦ������ͳ���ʯ��ˮ�����Ħ�����е�������ȷʵ��̼�������ط�Ӧ�Ļ�ѧ����ʽΪ�� ���� ��  �鿴�𰸺ͽ���>> ͬ����ϰ��� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |