
”¾ĢāÄæ”æŹµŃéŹŅÓŠ¼×ŅŅĮ½Ęæ¾ĆÖƵÄĒāŃõ»ÆÄĘ¹ĢĢå£¬Ä³Ń§Ļ°Š”×éĪŖĮĖŃŠ¾æĘä±äÖŹĒéæö£¬½ųŠŠĮĖČēĻĀŹµŃé: (µē×Ó³ÓŹ¾Źżµ„Ī»ĪŖæĖ)
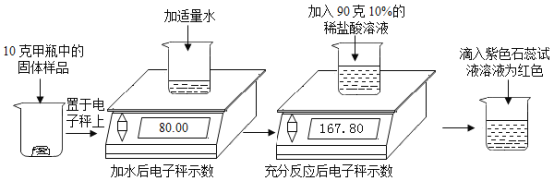
(1)µĪČė×ĻÉ«ŹÆČļŹŌŅŗŗóČÜŅŗĪŖŗģÉ«£¬ĖµĆ÷·“Ó¦ŗóČÜŅŗ³Ź____ŠŌ”£
(2)ÉĻŹöŹµŃéÖŠ²śÉśµÄ¶žŃõ»ÆĢ¼ĘųĢåÖŹĮæĪŖ______æĖ”£
(3)¼ĘĖć¼×Ęæ¹ĢĢåѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż____________”£
(4)ijĶ¬Ń§ĮķČ”10æĖŅŅĘæÖŠµÄ¹ĢĢåѳʷ,ÓĆ100æĖ15%µÄĻ”ĮņĖį°“Ķ¬Ńł·½·Ø½ųŠŠŹµŃ飬ĖūČĻĪŖ²»¹Ü¹ĢĢåѳʷ±äÖŹ³Ģ¶ČČēŗĪ£¬Ļ”ĮņĖį¼ÓČėŗ󣬶¼²»ŠčŅŖŹ¹ÓĆŹÆČļŹŌŅŗ£¬Ēė¼ĘĖćĖµĆ÷Ėū×ö³ö“ĖÅŠ¶ĻµÄŌŅņ__________”£
”¾“š°ø”æ Ėį 2.2 53% ÓĆ100g15%µÄĻ”ĮņĖį½ųŠŠŹµŃ飬Ļ”ĮņĖįŅ»¶Ø¹żĮ棬¹ŹĻ”ĮņĖį¼ÓČėŗ󲻊čŅŖ¼ÓŹÆČļŹŌŅŗ”£
”¾½āĪö”æ±¾Ģāæ¼²éĮĖøł¾Ż»Æѧ·½³ĢŹ½½ųŠŠ¼ĘĖć”£øł¾ŻÖŹĮæŹŲŗć·ÖĪöµĆµ½¶žŃõ»ÆĢ¼µÄÖŹĮ棬øł¾Ż¶žŃõ»ÆĢ¼µÄÖŹĮæ½įŗĻ»Æѧ·½³ĢŹ½¼ĘĖćѳʷ֊Ģ¼ĖįÄʵÄÖŹĮ棬½ųŅ»²½¼ĘĖć¹ĢĢåѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż”£
£Ø1£©×ĻÉ«ŹÆČļŹŌŅŗÓöĖįŠŌČÜŅŗ±äŗģÉ«£¬µĪČė×ĻÉ«ŹÆČļŹŌŅŗŗóČÜŅŗĪŖŗģÉ«£¬ĖµĆ÷·“Ó¦ŗóČÜŅŗ³ŹĖįŠŌ”£
£Ø2£©øł¾ŻÖŹĮæŹŲŗć£¬²śÉśµÄ¶žŃõ»ÆĢ¼ĘųĢåÖŹĮæĪŖ80.00g+90g-167.80g=2.2g£»
£Ø3£©Éč¹ĢĢåѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæĪŖx
Na2CO3+2HClØT2NaCl+H2O+CO2”ü
106 44
x 2.2g
![]() x=5.3g
x=5.3g
¹ĢĢåѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż=![]() ”Į100%=53%£»
”Į100%=53%£»
£Ø4£©ČōѳʷĶźČ«±äÖŹ£¬ŃłĘ·Č«²æ±ä³ÉĢ¼ĖįÄĘ”£
Éč£ŗÓė10gĢ¼ĖįÄĘ·“Ó¦ŠčĮņĖįµÄÖŹĮæĪŖy£¬
Na2CO3+H2SO4ØTNa2SO4+H2O+CO2”ü
106 98
10g y
![]() y=9.25g£¬ĖłŠčĻ”ĮņĖįµÄÖŹĮæ=
y=9.25g£¬ĖłŠčĻ”ĮņĖįµÄÖŹĮæ=![]() =61.64g”£61.64g<100g£¬Ļ”ĮņĖį¹żĮ棻
=61.64g”£61.64g<100g£¬Ļ”ĮņĖį¹żĮ棻
Čōѳʷƻӊ±äÖŹ£¬ŃłĘ·ÖŠÓŠ10gµÄĒāŃõ»ÆÄĘ”£
Éč£ŗÓė10gĒāŃõ»ÆÄĘ·“Ó¦ŠčĮņĖįµÄÖŹĮæĪŖz£¬
2NaOH+H2SO4=Na2SO4+2H2O
80 98
10g z
![]() z=12.25g£¬ĖłŠčĻ”ĮņĖįµÄÖŹĮæ=
z=12.25g£¬ĖłŠčĻ”ĮņĖįµÄÖŹĮæ=![]() =81.67g”£81.67g<100g£¬Ļ”ĮņĖį¹żĮ棻
=81.67g”£81.67g<100g£¬Ļ”ĮņĖį¹żĮ棻
ĖłŅŌ²»¹Ü¹ĢĢåѳʷ±äÖŹ³Ģ¶ČČēŗĪ£¬¼ÓČė100g15%Ļ”ĮņĖįŗó£¬Ļ”ĮņĖįŅ»¶Ø¹żĮ攣Ėū×ö³ö“ĖÅŠ¶ĻµÄŌŅņŹĒÓĆ100g15%µÄĻ”ĮņĖį½ųŠŠŹµŃ飬Ļ”ĮņĖįŅ»¶Ø¹żĮ棬¹ŹĻ”ĮņĖį¼ÓČėŗ󲻊čŅŖ¼ÓŹÆČļŹŌŅŗ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŃõĘųŹĒČĖĄąĪŽ·ØĄėæŖµÄĪļÖŹ£¬ŌŚ²»Ķ¬µÄÉś»ī”¢Éś²ś»·¾³ÖŠ¶ŌŃõĘųÅØ¶ČµÄŅŖĒó²»Ķ¬£¬ČĖĄą³£²ÉÓĆ²»Ķ¬µÄ·½·ØÖĘČ”ŃõĘų”£
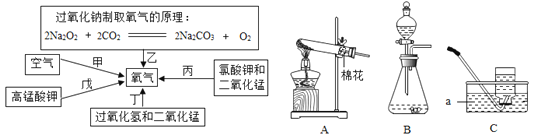
£Ø1£©¹¤ŅµÉĻŅ»°ć²ÉÓĆ¼×Ā·¾¶·ÖĄė¾»»ÆŗóµÄŅŗĢ¬æÕĘųÖĘČ”ŃõĘų”£ŌŚÉś²śÖŠÓ¦æŲÖĘĪĀ¶ČµÄ·¶Ī§ŹĒ___£»£ØŅŃÖŖŌŚ1.0l”Į105PaŹ±£¬O2µÄ·ŠµćĪŖ£183”ę£¬N2µÄ·ŠµćĪŖ£196”ę£©
£Ø2£©ŹµŃéŹŅ³£Ķعż±ū”¢¶””¢ĪģČżÖÖĀ·¾¶Ą“ÖĘČ”ŃõĘų£»
¢ŁŠ“³ö×°ÖĆCÖŠaŅĒĘ÷µÄĆū³Ę___£»
¢ŚČōÓĆĀ·¾¶Īģ£¬Ó¦Ń”ŌńµÄ·¢Éś×°ÖĆŹĒ____£»
¢ŪČōÓĆĀ·¾¶¶”£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ___£»
¢ÜŌŚÕāČżÖÖĀ·¾¶ÖŠĻūŗÄÄÜŌ“×īÉŁµÄŹĒ___£ØĢīŠņŗÅ£©£»
£Ø3£©ŌŚÉĻŹöĪåøöĀ·¾¶ÖŠ×īŹŹŗĻĒ±Ė®Ķ§ÖŠ»ńµĆŃõĘųµÄŹĒ___£ØĢīŠņŗÅ£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÕĘĪÕŹµŃéŹŅÖĘČ”³£¼ūĘųĢåµÄ·½·ØŹĒ³õÖŠÉś±Ų±øµÄ»ÆѧĖŲŃų£¬ŹµŃéŹŅĄļĻÖÓŠĀČĖį¼Ų”¢¶žŃõ»ÆĆĢ”¢ŠæĮ£”¢Ļ”ĮņĖį”¢ŹÆ»ŅŹÆŗĶĻ”ŃĪĖį£¬ŅŌ¼°ĻĀĮŠŅĒĘ÷£ŗ
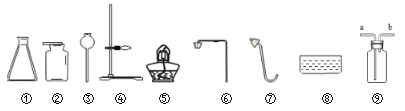
£Ø1£©ĄūÓĆÉĻŹöŅĒĘ÷æÉŅŌÖĘČ”¶žŃõ»ÆĢ¼£¬ÄćŃ”ŌńµÄŅĒĘ÷ŹĒ_____£ØĢīŠņŗÅ£©”£
£Ø2£©Čō²¹³äŅ»ÖÖŅĒĘ÷_____£ØĢīŅĒĘ÷Ćū³Ę£©£¬ŌŁĄūÓĆÉĻŹöĢį¹©µÄŅ©Ę·ŗĶŅĒĘ÷»¹æÉŅŌÖĘČ”ŃõĘų£¬ÄćŃ”ŌńµÄŅĒĘ÷ŹĒ¢Ü¢ŻŗĶ_____£ØĢīŠņŗÅ£©£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_____”£
£Ø3£©ŹµŃéŹŅĶس£ÓĆŠæĮ£ŗĶĻ”ĮņĖįÖĘČ”ĒāĘų£¬ČōÓĆČēĶ¼¢į×°ÖĆ²ÉÓĆÅÅæÕĘų·ØŹÕ¼ÆĒāĘų£¬ŌņĒāĘųÓ¦“Ó¶Ė½ųČė______£ØĢīa»ņb£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĶ¼ÖŠµÄ¢Ł”¢¢Ś·Ö±šŹĒ·śŌŖĖŲ”¢øĘŌŖĖŲŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄŠÅĻ¢£¬A”¢B”¢C”¢D·Ö±šŹĒĖÄÖÖĮ£×ӵĽį¹¹Ź¾ŅāĶ¼”£øł¾ŻĢāÖŠŠÅĻ¢»Ų“š£ŗ
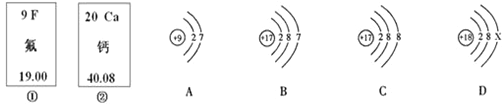
(1) ·śŌ×ÓµÄŗĖµēŗÉŹżĪŖ____________£¬
(2) øĘŌŖĖŲµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ________£»
(3) A”¢B”¢C”¢DÖŠŹōÓŚĶ¬ÖÖŌŖĖŲµÄĮ£×ÓŹĒ_____________(ĢīŠņŗÅ)£»
(4) AĮ£×ӵĻÆѧŠŌÖŹÓėB”¢C”¢DÖŠÄÄŅ»ÖÖĮ£×ӵĻÆѧŠŌÖŹĻąĖĘ_______(ĢīŠņŗÅ)£»
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŗĻ³ÉĘųŹĒ¹¤ŅµÉś²śÖŠµÄŅ»ÖÖŌĮĻĘų£¬Ö÷ŅŖ³É·ÖŹĒŅ»Ńõ»ÆĢ¼ŗĶĒāĘų”£ĖüæÉŅŌŅ±Į¶øÖĢś”¢Éś²ś¶ž¼×ĆѵȔ£Ēėøł¾ŻĶ¼Ź¾»Ų“š”£

(×¢£ŗĶ¼ÖŠĄØŗÅÄŚ»ÆѧŹ½±ķŹ¾ĻąÓ¦ĪļÖŹµÄÖ÷ŅŖ³É·Ö)
(1)ĒėŠ“³öĄūÓĆŗĻ³ÉĘųĮ¶ĢśµÄ»Æѧ·½³ĢŹ½________(Š“Ņ»øö¼“æÉ)”£
(2)¶ž¼×ĆŃ(CH3OCH3)±»³ĘĪŖ21ŹĄ¼ĶŠĀŠĶČ¼ĮĻ£¬ÄÜŹµĻÖøߊ§Ēå½ąČ¼ÉÕ£¬ĒėŠ“³ö¶ž¼×ĆŃŌŚæÕĘųÖŠ³ä·ÖČ¼ÉÕÉś³É¶žŃõ»ÆĢ¼ŗĶĖ®µÄ»Æѧ·½³ĢŹ½______”£
(3)ŗĻ³ÉĘųŌŚ²»Ķ¬“߻ƼĮ×÷ÓĆĻĀ£¬æÉŅŌŗĻ³É²»Ķ¬µÄĪļÖŹ”£½öÓĆŗĻ³ÉĘųĪŖŌĮĻ²»æÉÄܵƵ½µÄĪļÖŹŹĒ_______(Ģī×ÖÄøŠņŗÅ)
A£®¼×“¼(CH3OH) B£®ŅŅ¶žČ©(HC2O2) C£®ÄņĖŲ[CO(NH2)2]
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”抔øÕĶ¬Ń§ŌŚŃ§Ļ°»ÆѧÖŖŹ¶ŗó£¬ÖŖµĄ½ųČė¾ĆĪ“æŖĘōµÄ²Ė½ŃæÉÄÜŌģ³ÉĖĄĶö”£ÓŚŹĒĖūŗĶŠ”ĒæŅ»Ęš¶Ō×Ō¼Ņ¾ĆĪ“æŖĘōµÄ²Ė½ŃÄŚµÄĘųĢå³É·Ö½ųŠŠĮĖĢ½¾æ”£
£ØĢį³öĪŹĢā£©²Ė½ŃÄŚĘųĢåµÄ³É·ÖŹĒŹ²Ć“£æ
£Ø²éŌÄ׏ĮĻ£©Źß²ĖŌŚŅ»¶ØĢõ¼žĻĀ·¢½ĶÉś³ÉĘųĢ壬Ęä³É·ÖæÉÄÜŗ¬ÓŠCO2”¢CH4µČ”£
£Ø²ĀĻėÓė¼ŁÉč£©Š”øÕČĻĪŖ²Ė½ŃÄŚµÄĘųĢåŗ¬ÓŠCO2ŗĶCH4”£
£ØŹµŃéĢ½¾æ£©ĖūĆĒ¶Ō²Ė½ŃÄŚµÄĘųĢå½ųŠŠČ”Ńł°“Ķ¼ĖłŹ¾×°ÖĆ½ųŠŠŹµŃé

£Ø1£©Čō×°ĮæAÖŠ³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£¬Ö¤Ć÷ĘųĢåѳʷ֊ŗ¬ÓŠ__£»
£Ø2£©×°ÖĆBÄŚ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__£»
£Ø3£©×°ÖĆDÄŚÅØĮņĖįµÄ×÷ÓĆŹĒ__£»
£Ø4£©E“¦¹Ū²ģµ½øÉŌļĄäÉÕ±ÄŚ±ŚÓŠ__³öĻÖ£¬“ż»šŃęĻØĆšŗ󣬰ŃÉÕ±ŃøĖŁµ¹×Ŗ¹żĄ“£¬Į¢æĢĻņÉÕ±ÄŚµ¹ČėÉŁĮæ³ĪĒåŹÆ»ŅĖ®²¢Õńµ“£¬ŹÆ»ŅĖ®±ä»ė×Ē£¬Ö¤Ć÷ĘųĢåѳʷ֊ŗ¬ÓŠ__”£E“¦ĘųĢåČ¼ÉյĻÆѧ·½³ĢŹ½ĪŖ__”£
£ØŹµŃé½įĀŪ£©ĘųĢåѳʷ֊ŗ¬ÓŠCO2ŗĶCH4£¬Š”øյIJĀĻėÕżČ·”£
£Ø·“Ė¼ÓėĘĄ¼Ū£©
£Ø5£©Š”Ēæ¹Ū²ģÉĻŹöŹµŃé×°ÖĆ·¢ĻÖ£ŗ×°ÖĆAŗĶ×°ÖĆCĶźČ«ĻąĶ¬£¬ĖūČĻĪŖæÉŅŌČ”Ļū×°ÖĆC£®ÄćŹĒ·ńČĻĶ¬__£ØĢī”°ČĻĶ¬”±»ņ”°²»ČĻĶ¬”±£©ĄķÓÉŹĒ__”£
£Ø6£©øł¾ŻÉĻŹöŹµŃéĢ½¾æ½į¹ū£¬ĘųĢåѳʷ֊ŗ¬ÓŠCO2£¬ÓÉÓŚ¶žŃõ»ÆĢ¼__£¬ĖłŅŌ½ųČė¾ĆĪ“æŖĘōµÄ²Ė½ŃĒ°£¬Ó¦Ļņ²Ė½ŃÄŚĶØČė“óĮæµÄæÕĘų”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŅ»¶ØĢõ¼žĻĀ£¬ŌŚĆܱÕČŻĘ÷ÖŠ·¢ÉśÄ³·“Ó¦£¬·“Ó¦Ē°ŗóø÷ĪļÖŹµÄÖŹĮæČēĻĀĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ( )
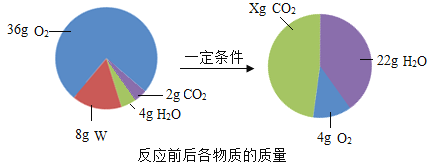
A. xµÄÖµĪŖ22 B. øĆ·“Ó¦ĪŖÖĆ»»·“Ó¦
C. WÓÉĢ¼”¢ĒāĮ½ÖÖŌŖĖŲ×é³É D. WÖŠĢ¼ŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ85£„
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
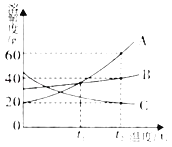
”¾ĢāÄæ”æČēĶ¼ŹĒA”¢B”¢CČżÖÖĪļÖŹµÄČܽā¶ČĒśĻߣ¬»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©A”¢BĮ½ÖÖĪļÖŹŌŚ_____”ꏱČܽā¶ČĻąĶ¬”£
£Ø2£©t2”ꏱ£¬BĪļÖŹµÄČܽā¶ČŹĒ_____”£
£Ø3£©t2”ꏱ£¬µČÖŹĮæµÄČżÖÖ±„ŗĶČÜŅŗÖŠČܼĮÖŹĮæ×īÉŁµÄŹĒ_____”£
£Ø4£©t2”ꏱ£¬½«ČżÖÖĪļÖŹø÷ag·Ö±š¼ÓČėµ½100gĖ®ÖŠ£¬³ä·ÖČܽāŗó£¬ČōÖ»ÓŠŅ»ÖÖĪļÖŹÄÜŠĪ³É±„ŗĶČÜŅŗ£¬ŌņaµÄȔֵ·¶Ī§ŹĒ_____”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŃ”ŌńŅĒĘ÷ĻĀ·½µÄ×ÖÄøĢīŠ“ŌŚĻąÓ¦ŗįĻßÉĻ£®

(1)ÓĆĄ“ĪüČ”ŗĶµĪ¼ÓÉŁĮæŅŗĢåµÄŅĒĘ÷ŹĒ______£»
(2)æÉŅŌÖ±½Ó¼ÓČȵÄŅĒĘ÷ŹĒ______£»
(3)ŹµŃéŹŅ³£ÓĆµÄ¼ÓČČŅĒĘ÷ŹĒ________£»
(4)ÅÅĖ®¼ÆĘų·Ø»įÓƵ½µÄŅĒĘ÷ŹĒ_______£»
(5)ĮæČ”ŅŗĢåŹ±£¬ŠčŅŖÓĆ_______£»
(6)ÉŁĮæČÜŅŗĻą»„·¢Éś·“Ó¦Ź±£¬ŠčŅŖÓĆ______£®
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com