
 K2MnO4+MnO2+O2���������ݴӼ���ƿ���ݳ�ʱ��˵�������Ѿ��ռ�����ʣ������У�ֻ�ж������̲�����ˮ��ͨ�������ܽ⡢���ˡ�ϴ�Ӹ���Ȳ������ɻ��ն������̣�
K2MnO4+MnO2+O2���������ݴӼ���ƿ���ݳ�ʱ��˵�������Ѿ��ռ�����ʣ������У�ֻ�ж������̲�����ˮ��ͨ�������ܽ⡢���ˡ�ϴ�Ӹ���Ȳ������ɻ��ն������̣� K2MnO4+MnO2+O2���� �����ݴӼ���ƿ���ݳ��� ���ˣ�
K2MnO4+MnO2+O2���� �����ݴӼ���ƿ���ݳ��� ���ˣ�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ̩���к��������꼶�п���ģ��ѧ�Ծ����������� ���ͣ�̽����
��1����ͬѧΪ̽��������ȼ�յ���������ͬʱ��ȼСľ����Сú�飬����Сú���ȼ����ʱ�䳤��ԭ���� ��
��2����ͬѧ�ڵ�ΰ�Ŀ�Ѩ�еμ�����������Һ���ٵμ���ɫ��̪��Һ���۲������ð�ɫ��ΰ�����Թܽ������ʵ����ŵ��� ��һ�㼴�ɣ���
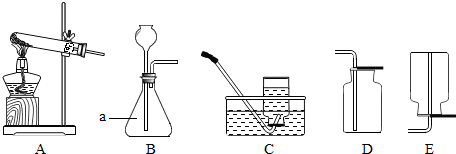
��3��������ͼ��ʾ��ȡ�����װ�ã��ش��������⣨װ��ѡ���������д����

��a������������ ��
����ʯ��ʯ��ϡ������ȡ������̼ʱ��ѡ�õķ������ռ�װ���� ����Ӧ�Ļ�ѧ����ʽΪ ��
�ۼ��ȸ��������ȡ��������Ӧ�Ļ�ѧ����ʽΪ ������Cװ���ռ��������۲쵽 ����ʾ������������Ӧ��ɺ�Ҫ��ʣ������л��ն������̣�Ӧ�����ܽ⡢ ��ϴ�Ӹ���Ȳ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ̩���к��������꼶�п���ģ��ѧ�Ծ��������棩 ���ͣ�̽����
��1����ͬѧΪ̽��������ȼ�յ���������ͬʱ��ȼСľ����Сú�飬����Сú���ȼ����ʱ�䳤��ԭ���� ��
��2����ͬѧ�ڵ�ΰ�Ŀ�Ѩ�еμ�����������Һ���ٵμ���ɫ��̪��Һ���۲������ð�ɫ��ΰ�����Թܽ������ʵ����ŵ��� ��һ�㼴�ɣ���
��3��������ͼ��ʾ��ȡ�����װ�ã��ش��������⣨װ��ѡ���������д����
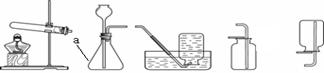

��a������������ ��
����ʯ��ʯ��ϡ������ȡ������̼ʱ��ѡ�õķ������ռ�װ���� ����Ӧ�Ļ�ѧ����ʽΪ ��
�ۼ��ȸ��������ȡ��������Ӧ�Ļ�ѧ����ʽΪ ������Cװ���ռ��������۲쵽 ����ʾ������������Ӧ��ɺ�Ҫ��ʣ������л��ն������̣�Ӧ�����ܽ⡢ ��ϴ�Ӹ���Ȳ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�꽭��ʡ̩���к������п���ѧ��ģ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com