
ú���ۺ������ǽ�ú����������ǿ�ȣ�ʹú�ֽ���������õ����ʣ�ú��������һ�֡�ú������Ҫ�ɷ���ʲô�أ�ij��ȤС��Ϊ��չ����̽����
���������ϡ���1��ú���п��ܺ���CO��CO2��H2��CH4�е�һ�ֻ��֡�
��2�������£��Ȼ���(PdCl2)��Һ����CO ʱ�����Ļ�ѧ��Ӧ����ʽΪ��
CO ��PdCl2��H2O 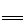 CO2��Pd��(��ɫ) ��2HCl
CO2��Pd��(��ɫ) ��2HCl
��ʵ�鷽������ȤС���ͬѧ�������װ��̽��ú���еijɷ֡�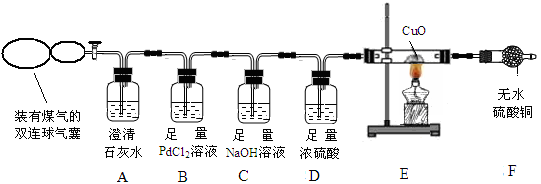
���������ۡ���ش�ʵ���е��й����⣺
��1��ʵ�鿪ʼ��A ������������˵��ú���в�����____________________��
��2����ú������CO ���ڣ���B�в����������� ��
��3��д��C �з�����Ӧ�Ļ�ѧ����ʽ___________________________����дһ�����ɣ�
D ��Ũ�����������_________________________��
��4����F ����ˮCuSO4����ɫ����E��һ�������Ļ�ѧ����ʽΪ ��
��5����ȼFװ�õ��������壬�л��������������Ϊú���к�CH4��������CH4����ȼ�գ�CH4ȼ�յĻ�ѧ����ʽ��____________________________________����ʦ����Ϊ����ȷ��ú���к���CH4��������__________________________��Ϊ��ȷ��ú�����Ƿ���CH4����ʦ���Ų�ȡ�IJ��������� ���۲쵽��������___________________________________���ɴ���֤ú����һ������CH4��
���������ۡ���1��CO2��2����Һ�в�����ɫ������3��NaOH+ HCl 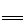 NaCl +H2O ��2NaOH+ CO2
NaCl +H2O ��2NaOH+ CO2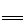 Na2CO3 +H2O ����������ȥ�����е�ˮ�֣�4��H2 +CuO
Na2CO3 +H2O ����������ȥ�����е�ˮ�֣�4��H2 +CuO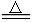 Cu +H2O ��5��CH4 +2O2
Cu +H2O ��5��CH4 +2O2 CO2 +2H2O ����ȷ��H2��Eװ���е�CuO �Ƿ���ȫ��Ӧ��F�����������п�����H2��ش������2 ��(�����𰸼��ɵ÷�) �ڻ����Ϸ���һ���ڱڸ��г���ʯ��ˮ���ձ�(�����𰸼��ɵ÷�) �ձ��ڱڳ���ʯ��ˮ�����(����һ���ɫ����)
CO2 +2H2O ����ȷ��H2��Eװ���е�CuO �Ƿ���ȫ��Ӧ��F�����������п�����H2��ش������2 ��(�����𰸼��ɵ÷�) �ڻ����Ϸ���һ���ڱڸ��г���ʯ��ˮ���ձ�(�����𰸼��ɵ÷�) �ձ��ڱڳ���ʯ��ˮ�����(����һ���ɫ����)
���������������1��ʵ�鿪ʼ��A ������������˵��ú���в����ڶ�����̼����2����ú������CO ���ڣ�����һ����̼�����ʾ��л�ԭ�Լ����Ͽ�֪��B�в�������������Һ�в�����ɫ��������3��д��C �з�����Ӧ�Ļ�ѧ����ʽNaOH+ HCl 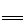 NaCl +H2O ��2NaOH+ CO2
NaCl +H2O ��2NaOH+ CO2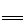 Na2CO3 +H2O ��D ��Ũ����������Ǹ���������ȥ�����е�ˮ�֣���4����F ����ˮCuSO4����ɫ��˵����ˮ���ɣ���E��һ�������Ļ�ѧ����ʽΪH2 +CuO
Na2CO3 +H2O ��D ��Ũ����������Ǹ���������ȥ�����е�ˮ�֣���4����F ����ˮCuSO4����ɫ��˵����ˮ���ɣ���E��һ�������Ļ�ѧ����ʽΪH2 +CuO Cu +H2O����5����ȼFװ�õ��������壬�л��������������Ϊú���к�CH4��������CH4����ȼ�գ�CH4ȼ�յĻ�ѧ����ʽ��CH4 +2O2
Cu +H2O����5����ȼFװ�õ��������壬�л��������������Ϊú���к�CH4��������CH4����ȼ�գ�CH4ȼ�յĻ�ѧ����ʽ��CH4 +2O2 CO2 +2H2O������ȷ��ú���к���CH4��������H2��Eװ���е�CuO �Ƿ���ȫ��Ӧ��F�����������п�����H2Ϊ��ȷ��ú�����Ƿ���CH4����ʦ���Ų�ȡ�IJ��������ڻ����Ϸ���һ���ڱڸ��г���ʯ��ˮ���ձ����۲쵽���������ձ��ڱڳ���ʯ��ˮ����ǣ��ɴ���֤ú����һ������CH4��
CO2 +2H2O������ȷ��ú���к���CH4��������H2��Eװ���е�CuO �Ƿ���ȫ��Ӧ��F�����������п�����H2Ϊ��ȷ��ú�����Ƿ���CH4����ʦ���Ų�ȡ�IJ��������ڻ����Ϸ���һ���ڱڸ��г���ʯ��ˮ���ձ����۲쵽���������ձ��ڱڳ���ʯ��ˮ����ǣ��ɴ���֤ú����һ������CH4��
���㣺���ʳɷ�̽��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ˮƿ�ǣ��д���CO2�����ݳ�������˵���������
| A�����ʼ��� | B��Ũ�ȼ�С |
| C���ܽ�ȼ�С | D����ΪCO2�IJ�������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���е���У������ʱ仯�ĽǶȷ�������Ҫ���ֻ�ѧ�仯���ǣ� ��
| A��˾�����Ҹ� | B������ɽ | C�����ճ�� | D������ĥ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
��һ����ɫҺ��A��һ�ֺ�ɫ����B����ܵõ���һ����ɫҺ��C����ɫ����D��E��D��ȼ�պ�����������ֻ����ʹ����ʯ��ˮ����ǵ�����F��
��1����д���������ʵ����ƣ�A B D F ��
��2����д��A��B�õ�C��D�����ֱ���ʽ�� ����B�� ���ã��˷�Ӧ���� ��Ӧ������ϡ��ֽ⡱����
��3����д��E+D��F�����ֱ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ������ϢϢ��أ����ˮ��̼�����ơ�ֲ���͡���ʯ�Ҽ��������У�ѡ���ʵ�������������и��⡣
����û������ܼ��� �� �����ڿ��������ɷ���������� �� ������
������ijЩʳƷ��������� ������������ˮ��ϲ������γ�����Һ���� ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������߲�¥��ʧ�����������Ķ����¼�ʱ�з�������������ʱ�����������У�
����ʪë����ס�ڱǡ���ǽ����������Ѱ�ҳ���������
�ڴ��Ŵ��������ȣ�
�����������䣬�ر��Ŵ��ȴ���Ԯ��
��Ѹ����¥������
������ȷ�ķ������� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧΪ�������Ŀɳ�����չ�����˾��ף�
��1������̫���ܷ����豸��̫������ˮ��һ����ֻҪ��һ��̫���ܰ����¥��������ͨ��̫���ܸ�����磮�ӻ�ѧ�Ƕȿ���̫������Ϊ����Դ����Ҫ�ŵ��У������㣩������ ���� ����
��2��������δ����������Դ��������Ϊ������������Ҫԭ�ϣ�Ҳ���������칤ҵ�ĸ���ȼ�ϣ���ȼ�յĻ�ѧ����ʽ���� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��100 mL����ͭ��Һ�м���һ���������ۣ���ַ�Ӧ����ˡ�����������ܳ��ֵ���( )
| A����Һ��ֻ��������ͭ���˳��Ĺ���ֻ���� |
| B����Һ��ֻ���������������˳��Ĺ���Ϊ����ͭ |
| C����Һ��ֻ��������ͭ���˳��Ĺ���ֻ��ͭ |
| D����Һ�к�����������������ͭ���˳��Ĺ���Ϊ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com