
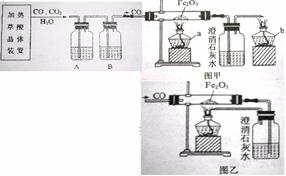
ij�С��ͬѧ�ü��Ȳ��ᾧ���Ƶõ�CO��H2C2O4·2 H2O==CO��+ CO2��+3H2O����������ԭ��ʵ�鲢����β���������������װ�ã�ͼ�ס�ͼ�ң���
�ش������й����⣺
��1���ڼ��ȷֽ���ᾧ���װ���У�������������ѡ��ʹ�ã�
���Թܢ��ձ��۾ƾ��Ƣ������������̨�������У������ܵ���Ƥ������ƿ
������Ҫ�õ������� ![]() ������ţ�
������ţ�
��2��Ϊ�˻�ø��﴿����CO��A��B��װ����Ӧ�ֱ��� ��
��3��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽ
��4��ѡ��ͼ�״���β����ʵ��ʱӦ�ȵ�ȼ ���a����b�������ľƾ��ơ�ͼ����ͼ����ʾ��β������������Ƚϣ�����Ҫ�ŵ���
��5���С���ͬѧͨ���������ϵ�֪�������£�CO�����Ȼ���PdCl2��Һ��Ӧ����������ѡ���˽�β��ֱ��ͨ���Ȼ�����Һ�н��д������÷�Ӧ�Ļ�ѧ����ʽΪ��CO+ PdCl2+H2O==Pd��+ CO2 + 2R,����R�Ļ�ѧʽΪ ���˷�������������ȳ��˾���ͼ�ұ�ͼ����ʾ�������ŵ��⣬��ʵ��Ƕȿ������е��ŵ���
��д��һ�������𰸼��ɣ�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�ʵ����
 CO��+ CO2��+3H2O����������ԭ��ʵ�鲢����β���������������װ�ã�ͼ�ס�ͼ�ң���
CO��+ CO2��+3H2O����������ԭ��ʵ�鲢����β���������������װ�ã�ͼ�ס�ͼ�ң���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
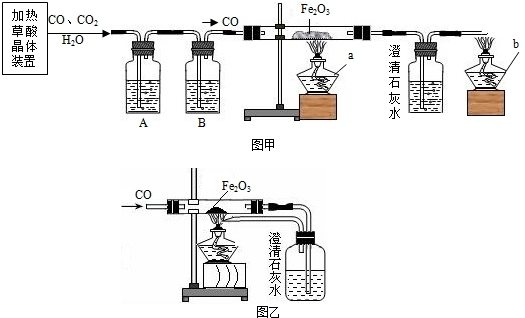
ij�С��ͬѧ�ü��Ȳ��ᾧ���Ƶõ�CO��H2C2O4·2 H2O==CO��+ CO2��+3H2O����������ԭ��ʵ�鲢����β���������������װ�ã�ͼ�ס�ͼ�ң���

�ش������й����⣺
��1���ڼ��ȷֽ���ᾧ���װ���У�������������ѡ��ʹ�ã�
���Թܢ��ձ��۾ƾ��Ƣ������������̨�������У������ܵ���Ƥ������ƿ
������Ҫ�õ��������� ������ţ�
��2��Ϊ�˻�ø��﴿����CO��A��B��װ����Ӧ�ֱ��� ��
��3��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽ
��4��ѡ��ͼ�״���β����ʵ��ʱӦ�ȵ�ȼ ���a����b�������ľƾ��ơ�ͼ����ͼ����ʾ��β������������Ƚϣ�����Ҫ�ŵ���
��5���С���ͬѧͨ���������ϵ�֪�������£�CO�����Ȼ���PdCl2��Һ��Ӧ����������ѡ���˽�β��ֱ��ͨ���Ȼ�����Һ�н��д������÷�Ӧ�Ļ�ѧ����ʽΪ��CO+ PdCl2+H2O==Pd��+ CO2 + 2R,����R�Ļ�ѧʽΪ ���˷�������������ȳ��˾���ͼ�ұ�ͼ����ʾ�������ŵ��⣬��ʵ��Ƕȿ������е��ŵ���
��д��һ�������𰸼��ɣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
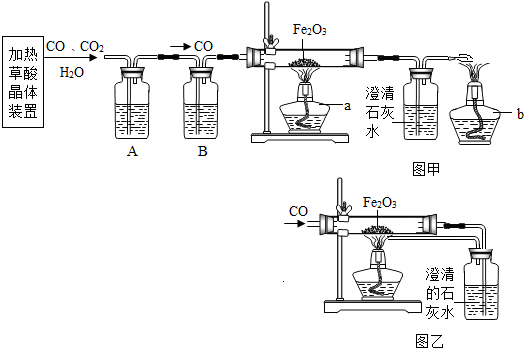
ij�С��ͬѧ�ü��Ȳ��ᾧ���Ƶõ�CO
��H2C2O4��2 H2O==CO��+ CO2��+3H2O����������ԭ��ʵ�鲢����β���������������װ�ã�ͼ�ס�ͼ�ң���
�ش������й����⣺
��1���ڼ��ȷֽ���ᾧ���װ���У�������������ѡ��ʹ�ã�
���Թܢ��ձ��۾ƾ��Ƣ������������̨�������У������ܵ���Ƥ������ƿ������Ҫ�õ��������������������������� ������ţ�
��2��Ϊ�˻�ø��﴿����CO��A��B��װ����Ӧ�ֱ������� ������
��3��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽ��������������������������
��4��ѡ��ͼ�״���β����ʵ��ʱӦ�ȵ�ȼ���� ���a����b�������ľƾ��ơ�ͼ����ͼ����ʾ��β������������Ƚϣ�����Ҫ�ŵ�����
������������
��5���С���ͬѧͨ���������ϵ�֪�������£�CO�����Ȼ���PdCl2��Һ��Ӧ����������ѡ���˽�β��ֱ��ͨ���Ȼ�����Һ�н��д������÷�Ӧ�Ļ�ѧ����ʽΪ��CO+ PdCl2+H2O==Pd��+ CO2 + 2R,����R�Ļ�ѧʽΪ���������� ���˷�������������ȳ��˾���ͼ�ұ�ͼ����ʾ�������ŵ��⣬��ʵ��Ƕȿ������е��ŵ������������������������������� ��д��һ�������𰸼��ɣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com