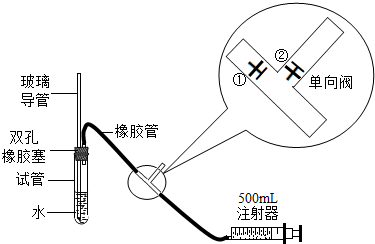
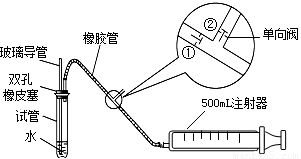
ע������ԭ��˵������ע�����ƻ���ʱ���ٹرգ��ڴ���ע����������ʱ���ٴ��ڹرգ�
��1���ס�����ͬѧ������������װ�ü�������ԣ���ͬѧ��������ס�Թܣ������Թܣ����۲쵼�����Ƿ���Һ�������������Ƿ��ܣ���
�����ܻ��ܣ���
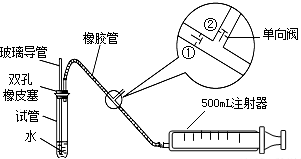
��ͬѧ���ȴӲ����������Թ���ע��ˮ���۲쵼����Һ�����Թ���Һ���Ƿ��γɸ߶Ȳ����һ��ʱ��۲�Һ����Ƿ�仯�ķ������Ƿ��ܣ�
�����ܻ��ܣ���
���������ַ��������ܣ���˼���·������������пհף����������У��������пհף��������Թ���װ������ˮ����֤�������ܵ��¶˽�û��ˮ�У���Ȼ�������������
������
����ʵ������֤����װ�õ����������ã�
��2�����Թ��м���0��00127%�ĵ�ˮ10g��������������ˮϡ�ͺ��ټ���2��3�ε�����Һ�����ó���ҺA���ⶨָ���ص������SO
2�ĺ���ʱ������ע�����Ļ��������������Թ��з����Ļ�ѧ��Ӧ����ʽΪSO
2+I
2+2H
2O=H
2SO
4+2HI��A��Һ��
ɫ��Ϊ
ʱ������Ӧǡ����ȫ���У���ʱӦֹͣ������
��3���ҹ����������������ж�ÿ�ο��������ⶨ��SO
2�����Ũ����ֵ��mg/m
3����һ������0.15����������0.50����������0.70���ÿ���С��ֳɵ�һ�С��͵ڶ��С�飬ʹ����ͬ��ʵ��װ�ú���ҺA����ͬһ�ص㡢ͬʱ����������SO
2�ĺ���������Ӧǡ����ȫ���У���¼����ʱ��ʹ������£��ٶ�ÿ�γ���500mL�����뽫�±���д����������ʱ����2λ��Ч���֣���
��4��ͨ���ⶨ�����㣬���жϳ�����ص�Ŀ�����S0
2�������
�������3�����о�
| ���� |
��һС�� |
�ڶ�С�� |
| ����ʱ�� |
20���� |
21���� |
| �������� |
100 |
130 |
| SO2������mg/m3 |
|
|
��һС�飺
���ڶ�С�飺
��
����ʵ������У��軺���鶯������Ŀ����
�������ٳ�������ⶨ�Ľ����
����ƫ��ƫС����Ӱ�죩��
���жϸõص�Ŀ�����SO
2��������
�������֣�������
�����һ���ڶ�����С��IJⶨ���ȷ����һС��ʵ���������ϴ�ƫ���ԭ���ǣ�����С������ҩƷ��װ�þ������⣩
��




 �������ϵ�д�
�������ϵ�д�