
��۲�����װ�ò��ش�������⣮
��1����������c ���������� ����
��2��A װ���з�����Ӧ�Ļ�ѧ����ʽ���� ����
��3�����ø��������ȡ������Ӧѡ�õķ���װ������ �������Ӧ�ı�ţ����仯ѧ����ʽΪ�� ��������Dװ���ռ������������ķ������� ����
��4��F װ�ô�a��b ��ijһ���ӿڽ��������Դ���D��E װ���ռ����壬���ҿ��Լ�������������е��ݳ�������B��Fװ������ȡ���ռ�������̼���壬����B װ�õ�Ӧ���� ���ڣ�ѡ�a����b������
��1������©������2��CaCO3+2HCl=CaCl2+H2O+CO2����
��3��C�� 2KMnO4 K2MnO4+MnO2+O2������������ľ������ƿ�ڣ���������ľ����ȼ��������ȼ�գ���֤��������������4��a��
K2MnO4+MnO2+O2������������ľ������ƿ�ڣ���������ľ����ȼ��������ȼ�գ���֤��������������4��a��
���������������1������c������Һ��ij���©������2������ʯ��ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼������ʽ�ǣ�CaCO3+2HCl=CaCl2+H2O+CO2������3�����ȸ��������ȡ�������ڹ�������ͣ���ѡ����װ��C�����ȸ��������������ء��������̺�����������ʽ�ǣ�2KMnO4 K2MnO4+MnO2+O2��������Dװ���ռ������������ķ����ǽ������ǵ�ľ�����ڼ���ƿ�ڣ��۲��Ƿ�ȼ������������4��������̼���ܶȱȿ�����Ӧ�ӳ����ܽ�������������ѹ������ƿ�ϲ��ų���
K2MnO4+MnO2+O2��������Dװ���ռ������������ķ����ǽ������ǵ�ľ�����ڼ���ƿ�ڣ��۲��Ƿ�ȼ������������4��������̼���ܶȱȿ�����Ӧ�ӳ����ܽ�������������ѹ������ƿ�ϲ��ų���
���㣺��������ķ���װ�ú��ռ�װ����ѡȡ������


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ͼ�ش����⡣ 
��1�������ٵ������� ���ڵ������� ��
��2��ʵ�����ø��������ȡ�����Ļ�ѧ����ʽΪ ��ѡ�õķ���װ���� ������ĸ��ţ���ͬ����ʵ�����ù���������ȡ�����Ļ�ѧ����ʽΪ ��ѡ�õķ���װ���� ��
��3��ʵ������ʯ��ʯ��ȡ������̼�Ļ�ѧ����ʽΪ ��ѡ�õ��ռ�װ���� ����ȼ�ŵ�ľ������ƿ�ڣ����۲쵽 ��˵��ƿ���ѳ���������̼����ü���ƿ�м�����ɫʯ����Һ���۲쵽��Һ�� ɫ��������Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ���е�ʵ��������飬�ƶ����ĸ���ǩ���� O2����ȡ����CO2����ȡ��������50g10%��NaCl��Һ���ܴ����ᴿ��ÿ��ͬѧ��ȡһ����ǩ����ʵ�顣
��1����ͬѧ��ǩ������ʵ���ң����ֱ���ʵ��������Ҫ��������������ҩƷ��
����ͼ������D������Ϊ ��
�ڼ�ͬѧ�鵽�Ŀ�ǩӦ���� ����ʵ�����ƣ�����ʵ���ԭ��Ϊ ���û�ѧ����ʽ��ʾ����Ӧѡ�õ������� ������ĸ���ţ���
��2����ͬѧ����ɡ�����50g 10%��NaCl��Һ��ʵ������У�ͨ�����㣬�����NaCl g���ܽ�NaClʱ�õ��IJ��������������� ��
��3����ͬѧ�鵽�Ŀ�ǩΪ�ø���������������䷴Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͼ1��ʵ���ҳ��õ�װ�ã���ش��������⣺
��1��ʵ����������غͶ������̻����ȡ�����ķ���װ������ ��������ţ�����Ӧ�Ļ�ѧ����ʽΪ�� ����
��2��д��ʵ�����ô���ʯ��ϡ���ᷴӦ��ȡ������̼�Ļ�ѧ����ʽ�� �����ռ�CO2Ӧѡ�õ�װ������ ��������ţ���
��3������ͼ2��ʾװ����H2����������H2�������ͨ����Һ©������һ�������ϡ���ᣬ��Բ����ƿ��ʢ�ŵ�0.65gп����ַ�Ӧ��ϡ������������
��װ�ýӿڵ�����˳��Ϊ��a���� ����d���b��c����c��b������
��������0.65gп������ϡ���ᷴӦ�ڸ�������Ӧ���ռ���224mL��H2����3��ʵ�������H2�����ƽ��ֵԼΪ239mL������ʵ�������ȷ���淶����ɸ�������Ҫԭ������ ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ͼ����ʾװ�ûش��������⣺
��1��д��ͼ�б�ʾ���������ƣ����� ����
��2��ʵ������KMnO4��ȡO2��Ӧѡ�õķ���װ��Ϊ�� ������ĸ��ţ���ͬ�����ռ�װ�ÿ�ѡ���� ����дһ�ּ��ɣ���ȡ��KMnO4����ʱ��Ӧѡ�õ�����������д���÷�Ӧ�Ļ�ѧ����ʽ�� ����
��3��ע����C�����ڼ��װ��E�������ԣ��������£�
������ƿ�м�������ˮ��û������©���¶˴����ڽ�ע����C���ӵ�װ��E�ĵ��ܿڴ���
�ۻ�������ע����C�Ļ������۲쵽�� ������ʾװ��E�����������ã�
��4��ʵ������װ��E��ȡCO2ʱ������ע����C�滻����©�����ŵ����� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ��ȤС��ѡ����ͼ��ʾװ�ã������й��������ȡ������ʵ�飬���
���������⣺
A B C D E F G H
��1��д���б���������ƣ��� ���� ��
��2��ѡ��Aװ����ȡ�����Ļ�ѧ����ʽΪ ��ʵ��ʱ���Թܿڷ�һ�������������� ������ˮ���ռ������ĺ���ʱ���� ��
��3����B��F���ӣ���F�г��ֵ������� ��ԭ���� ���û�ѧ����ʽ��ʾ������B��G���ӣ���G�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��4����B��H���ӣ���H�е͵�������Ϩ�𣬸ߵ������Ϩ���ɴ�˵����CO2�������� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������װ�ã��ش��������⣺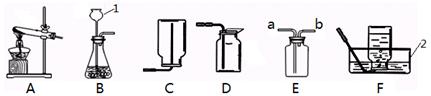
����д��ͼ�б�����ĸ���������ƣ�1�� 2��
��ʵ�����ø��������ȡ�������ռ�������������Ӧѡ�õ�װ������� ������ĸ����
��д���÷�Ӧ�ı���ʽ�� ��
ʵ��ʱ��װ���Թܿ�Ӧ��һ��������Ŀ���� ��
�ø�װ���ռ���������ʱ���� �� ����ѡ��ٽ������Ƴ�ˮ�� ��Ϩ��ƾ��ƣ�
��������Ŀ���� ��
��ʵ�����ù���������Һ��ȡ���������õķ���װ���� ����д����Ӧ�ı���
ʽ�� ������Dװ���ռ���������д��������
������ ��
�ܲ������ϵ�֪������(NH3)��һ���ܶȱȿ���С�Ҽ�������ˮ�����塣����ѡ�ü����Ȼ�狀��������ƵĹ�����������ȡ����ʱ����ѡ��ķ���װ���� ��
�������Eװ���ռ������������ӵ��� ���a����b������ͨ�롣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������г��õ�������װ�ã��ش�������⣨4�֣��� 
��1��д������c�����ƣ� d�����ƣ�
��2����������ȡһ�����Һ��������� ������ţ�
��3��ʵ������˫��ˮ�Ͷ���������ȡ�������ռ�������װ�ÿ�ѡ�� ������ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͨ���������µĻ�ѧѧϰ���������Ѿ�����������ʵ������ȡһЩ�������֤�������ʵ��й�֪ʶ������ͼʾ�ش�����
��1��д��ͼ�б�ʾ���������ƣ��� ���� ��
��2��ʵ����������غͶ���������ȡ����Ӧѡ�õķ���װ��Ϊ ������ĸ��ţ���ͬ����O2����ͼ��F��Gװ���ռ�����Fװ���ռ�������Ϊ________________��������________________������ʱ��˵������������д���÷�Ӧ�Ļ�ѧ����ʽ __________ ��
��3��ʵ���ҳ��ù���������Һ��ȡ�������÷����������������ŵ㣬�磺___(дһ��)
��4��I��J����֤�������ʵIJ���ʵ�飬����ʵ���У�ʵ��ǰ����ƿ�ﶼװ��������ˮ�����У�I����ˮ�������� ��J����ˮ�������� ��I�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��5��ע����C�ڻ�ѧ�е����úܶ࣬�磺�����ڼ��װ��E�������ԡ��������£�������ƿ�м�������ˮ��û����©���¶ˡ���ע����C���ӵ�װ��E�ĵ��ܿڴ��������ƶ�ע����C�Ļ��������װ��E�����������ã��۲쵽�������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com