
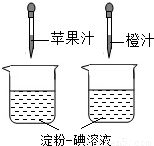
������Һ��һ�ֳ��õĻ�ѧ�Լ�����������ˮ����ɫ����ͼ��ʾ������ά����
C��ʹ���ۣ�����Һ��ɫ�����ʣ����ԱȽ�ƻ��֭�ͳ�֭��ά����C�����Ķ��٣�Ҫ�ó���ȷ���ۣ�ʵ������в���Ҫ���п��Ƶ�������[
����]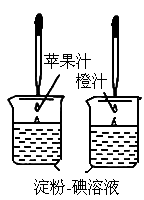
A
���ձ��Ĵ�С�������B
���ձ��еĵ���������Һ�������������C
����ͷ�ιܵγ���ÿ�ι�֭������������D
����ͷ�ιܵγ��Ĺ�֭�ĵ���������� ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | ͭ | ���� |
| ��� |

| ���� | ̿�ۣ�C�� | һ����̼��CO�� | ������H2�� | ���飨CH4�� | �Ҵ���C2H5OH�� |
| ״̬ | ���� | ���� | ���� | ���� | Һ�� |
| ������KJ�� | 392.8 | 282.6 | 285.8 | 890.3 | 1367 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
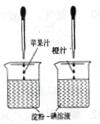 ��2004?�Ϻ�����1��������Һ��һ�ֳ��õĻ�ѧ�Լ�����������ˮ��
��2004?�Ϻ�����1��������Һ��һ�ֳ��õĻ�ѧ�Լ�����������ˮ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
(1)
������Һ��һ�ֳ��õĻ�ѧ�Լ�������ʹ��ˮ��________ɫ��(2)
��ͼ��ʾ������ά����C��ʹ���۵���Һ��ɫ�����ʣ����ԱȽ�ƻ��֭�ͳ�֭��ά����C�����Ķ��٣�Ҫ�ó���ȷ���ۣ�ʵ������в���Ҫ���п��Ƶ�������[
����]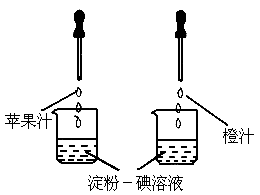
A
���ձ��Ĵ�С�������B
���ձ��еĵ���һ����Һ�������������C
����ͷ�ιܵγ���ÿ�ι�֭������������D
����ͷ�ιܵγ��Ĺ�֭�ĵ����������(3)
������Ҫ��Ӫ���أ��������ࡢά�����⣬����ˮ����֬�������ʺ�________�ȣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1�����ż�����������ռ��Ͳ��ϸ��£��ϵ����в��ϵĻ����������������ǵ����ӣ�д��������������ֳ��ò��ϵ������������ϡ����������ʲ��ϡ������߷��Ӳ��ϡ�����
| ���� | ͭ | ���� |
| ��� |
��2���ٵ�����Һ��һ�ֳ��õĻ�ѧ�Լ�����������ˮ��______ɫ��
����ͼ��ʾ������ά����C��ʹ����-����Һ��ɫ�����ʣ����ԱȽ�ƻ��֭�ͳ�֭��ά����C�����Ķ��٣�Ҫ�ó���ȷ���ۣ�ʵ������в���Ҫ���п��Ƶ�������______
A���ձ��Ĵ�С�������
B���ձ��еĵ���-����Һ�������������
C����ͷ�ιܵγ���ÿ�ι�֭������������
D����ͷ�ιܵγ��Ĺ�֭�ĵ����������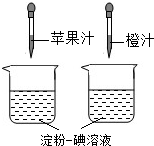
��������Ҫ��Ӫ���أ����˵��۵����ࡢά�����⣬����ˮ����֬�������ʺ�______�ȣ�
��3���±��Ǽ��ֳ���ȼ�ϣ�lmol����ȫȼ��ʱ�ų���������
| ���� | ̿�ۣ�C�� | һ����̼��CO�� | ������H2�� | ���飨CH4�� | �Ҵ���C2H5OH�� |
| ״̬ | ���� | ���� | ���� | ���� | Һ�� |
| ������KJ�� | 392.8 | 282.6 | 285.8 | 890.3 | 1367 |
�ٴ������Ƕȷ�����Ŀǰ���ʺϼ�ͥʹ�õ���������ȼ����______��
��д���ܵ�ú���е�һ����̼ȼ�յĻ�ѧ����ʽ______��lmolһ����̼���ȼ�գ����������������ʵ�����______moI��
�۳��ȼ��lmol���и���ȼ�ϣ��ŷų�������̼����������______��
�ܿ���ȼ�ϴ������ޣ�������ȼ�չ����л������Ⱦ��������Դ�������ķ�չս�ԣ��ҹ��������õ���ɫ��Դ�����ܡ�______�ȣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2004���Ϻ����п���ѧ�Ծ��������棩 ���ͣ������
| ���� | ͭ | ���� |
| ��� |
| ���� | ̿�ۣ�C�� | һ����̼��CO�� | ������H2�� | ���飨CH4�� | �Ҵ���C2H5OH�� |
| ״̬ | ���� | ���� | ���� | ���� | Һ�� |
| ������KJ�� | 392.8 | 282.6 | 285.8 | 890.3 | 1367 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com