
����ʵ������У����ȡ��롰��˳����ȷ����
A. �Ʊ�����ʱ����װҩƷ������װ�õ�������
B. ����ˮ���ռ����������Ȱѵ�������ˮ�棬��ֹͣ����
C. ����Ͳ��ȡ10mLҺ��ʱ���ȵ���ӽ�10mL��Һ�壬���ý�ͷ�ιܲ����̶���
D. ��������ƽ��������ʱ���ȼ�����������룬�ټ�����С�����룬����ƶ�����
A �������� �����Ʊ�����ʱ��Ӧ���ȼ��װ�õ������ԣ���װҩƷ���ʣ���������ˮ���ռ����������Ȱѵ�������ˮ�棬��ֹͣ���ȣ�Ŀ���Ƿ�ֹˮ������ը���Թܣ����ԣ���ȷ������Ͳ��ȡ10mLҺ��ʱ���ȵ���ӽ�10mL��Һ�壬���ý�ͷ�ιܲ����̶��ߣ���ֱֹ�ӵ�Һ�岻�ÿ����������ԣ���ȷ����������ƽ��������ʱ���ȼ�����������룬�ټ�����С�����룬����ƶ����룬�������ƽ��ʹ�ã����ԣ���ȷ����ѡ���� ... ������������ϵ�д�
������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2019����꼶���̰滯ѧȫ������µ�Ԫ���Ծ� ���ͣ���ѡ��
���������ɷ��ӹ��ɵ���
A. п B. ����ͭ C. C60 D. ���ʯ
C �������� Aѡ�����п������ԭ��ֱ�ӹ��ɵģ�Bѡ������ͭ�������ӹ��ɵģ�Cѡ��C60�����ɷ��ӹ��ɵģ�Dѡ����ʯ������ԭ��ֱ�ӹ��ɵģ��ʴ�ѡ��C�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2019����꼶��ѧ��³�̰滯ѧ�ϲ��1�²��뻯ѧ���õ�Ԫ����� ���ͣ���ѡ��
���б仯���������仯���ǣ� ��
A. ʯ�ҽ�Ĩǽ��ǽ�ڷ�Ӳ B. ��̪��Һ��������Һ��Ϊ��ɫ
C. �ÿ����Ƹ���O2��N2�� D. ����ʯ������ʯ��
C �������� A��ʯ�ҽ�Ĩǽ�����е����������ܺͿ����еĶ�����̼��Ӧ����̼��ƺ�ˮ�����ڻ�ѧ�仯������B����̪��Һ���Լ��Ե����ʷ�Ӧʱ�ܹ���ɫ�����ڻ�ѧ�仯������C���ÿ����Ƹ���O2��N2�ȹ�����û�����������ɣ����������仯����ȷ��D����ʯ�Һ�ˮ��Ӧ��������ʯ�ң����ڻ�ѧ�仯������ѡC���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2018-2019ѧ���һѧ���˽̰���꼶��ѧ�ϲ��1���߽���ѧ���絥Ԫ���Ծ� ���ͣ������
��ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ�����������ʵ��Ҫ����գ�
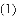
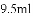
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2018-2019ѧ���һѧ���˽̰���꼶��ѧ�ϲ��1���߽���ѧ���絥Ԫ���Ծ� ���ͣ���ѡ��
��ѧ��ѧ�ķ�չ�ٽ�����������������������ǵ�����������������������������ص��ش����⣬���кͻ�ѧ������ص���
�ٻ������� ����Դ�������� �۹��ܲ����о� ����������̽���� ��
A. �٢� B. �ڢۢ� C. �٢ڢ� D. �٢ڢۢ�
D �������� ���ݻ�ѧ�о�������ͻ�ѧ����;���ش��⡣ ��ѧ���о����ʵ���ɡ��ṹ�����ʡ��Լ��仯���ɵĻ�����Ȼ��ѧ��Ҳ����˵��ѧ���о����ʵ�һ�ſ�ѧ��ͨ��������µĻ��������ʿ��Ա���������ͨ�������������Դ���Լ��ٶԻ�������Ⱦ��ͨ����������������о��²��ϣ���������̽����������ѧ�о������ݡ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡ2019����꼶��ѧ�ڰ˵�Ԫ���Ծ� ���ͣ�������
С����ⶨCu��Zn�Ͻ��Cu��Ag�Ͻ���ͭ������������ʵ����ֻ�ṩ��һƿϡ����ͱ�Ҫ��������
(1)����Ϊ���ܲ��ͭ�����������ĺϽ���_____�Ͻ�
(2)С��ȡ�úϽ�ķ�ĩ32.5 g�������ĸ������ַ�Ӧ���ⶨ������0.4 g���壬���������������úϽ���ͭ����������_____��
ͭп60% �������� (1)ֻ��������ͱ�Ҫ����������Ͻ����躬���������ᷴӦ�Ľ�����п�������ǰ�棬��ͭ����������ĺ��棬�������ᷴӦ������ֻ�ܲ��ͭп�Ͻ� (2)������0.4g������Ҫп������Ϊx���� �úϽ��е�Cu������������= ��100%=60%�� �𣺸úϽ���ͭ����������Ϊ60%���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡ2019����꼶��ѧ�ڰ˵�Ԫ���Ծ� ���ͣ������
��������Ӧ�ù㷺�����·���г��õ�������ͭ���������ȡ�
(1)����������ɽ�����Ⱦ���___________�����ԣ����������ӵ���Ԫ����
(2)��ͭƬ������������Һ�У�������Ӧ�Ļ�ѧ����ʽΪ___________________��
(3)��Ҫ��֤Cu��Ag��Fe�Ļ��ǿ������ѡ����Լ�����______(�����)��
A��Fe��Cu��Ag��ϡ���ᡡ����B��Cu��Ag��FeSO4��Һ������C��Fe��Ag��CuSO4��Һ
�۵��Cu��2AgNO3=Cu(NO3)2��2AgC �������� (1)�Ͻ�������ǣ�Ӳ�ȴ��۵�ͣ���ʴ�� (2)��CuƬ����AgNO3��Һ�У�ͭ����������Ӧ������������ͭ����Ӧ�Ļ�ѧ����ʽΪ��Cu+2AgNO3=2Ag+Cu��NO3��2�� (3)��Ҫ��֤Cu��Ag��Fe�Ļ��ǿ������ѡ����Լ����ǣ�Fe��Ag��CuSO4��Һ��������������ͭ��Һ�У����ı����к�ɫ������...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2018����꼶�п���ģ��ѧ�Ծ� ���ͣ���ѧ̽����
��ѧ̽����ѧϰ��ѧ��Ҫ����Ч��ѧϰ��ʽ��ij��ѧ��ȤС���ͬѧ�������Ϊר����������¼���̽��ʵ�顣

��1����ͼ1��ijͬѧ��Ƶ�̽������Ӧ��ʵ�飬��ش�
��д���÷�Ӧ�Ļ�ѧ����ʽ____________��
�ڸ�ʵ�����û��������������Ӧ�����Һ�еμӷ�̪��Һ����Һ����ɫ��Ҫȷ����Ӧ����Һ�ijɷ֣��ɹ�ѡ�õ��Լ��У�ʯ����Һ������ͭ���塢̼������Һ���Ȼ�����Һ������Ϊʲô�Լ����ܴﵽʵ��Ŀ�ģ�_______
��2���������ý������ᷴӦ��ʵ���У�ijͬѧ������������Һ������μӵ��������У��������Ҳ�����ݲ������������Ͽ�֪������������������Һ��Ӧ����������ƫ�����ƣ� NaAlO2�����û�ѧ����ʽΪ____________��
��3��ij��ѧС��ͨ����������Һ�н��е�ʵ�飬̽�����ֽⷴӦ������������
a��HCl��Ca��OH��2
b��H2SO4��K2CO3
c��NaOH��CuSO4
d��Ba��NO3��2��Na2SO4
e��Na2CO3��KOH
������ʵ���У����ܷ������ֽⷴӦ����___��
��ʵ��c�пɹ۲쵽��������________��
��4����ͼ2��ijͬѧ����150g������������Ϊ7%���Ȼ�����Һ�IJ������̡�
������������һ�����ô���ᵼ����������Һ��������������___7%������������������=����������������ȷ��˳��Ϊ_______��
��5��Ϊ�ⶨ���ֱ��ʵ��ռ����������Ƶ�������������ȡ�ù�����Ʒ10g������50gϡ�����У�ǡ����ȫ��Ӧ����Ӧ����Һ������Ϊ57.8g�����㣺����Ʒ���������Ƶ���������________��
H2SO4+2NaOH�TNa2SO4+2H2O���Ȼ�����Һ��2Al+2NaOH+2H2O�T2NaAlO2+3H2����e��������ɫ�����������ڢ٢ݢۢܣ�����Ʒ���������Ƶ���������47%�� �������� �������� (1)��ϡ�������������Ʒ������кͷ�Ӧ�����������ƺ�ˮ���÷�Ӧ�Ļ�ѧ����ʽ��H2SO4+2NaOH�TNa2SO4+2H2O���ڸ�ʵ�����û��������������Ӧ�����Һ�еμӷ�̪��Һ...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡ2019����꼶��ѧ��һ��Ԫ���Ծ� ���ͣ���ѡ��
�콨ͬѧ����ѧ���Ļ�ѧ֪ʶ�жϼ��еIJ������Ƿ�ϴ�Ӹɾ���������
A. �����ڱڿ��������� B. �����ϵ�ˮ������ˮ�Σ�Ҳ���ɹ�����
C. �ñ���װˮ��ˮ�dz������� D. ������������Ϊ�ж�����
B �������� ������еIJ������Ƿ�ϴ�Ӹɾ��������DZ����ϵ�ˮ������ˮ�Σ�Ҳ���ɹ����£����Ǹ���һ����ȵ�ˮĤ����ѡ�£��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com