
(8��)ij��ȤС�鷢��һ�������ɻ��������ϣ����ϱ��ϵijɷ��������ơ������ʳ�Ρ�����Ҫ̽���������ɻ��������ж�����Щ���ʶ�Ѽ�������á�����ȡ����������ˮ���ܽ⣬��ֽ������ˣ�ȡ��Һ̽����ɷ֡�
����������衿���Ƕ���Ϊ��Һ��һ����NaCl��NaOH�������������ƵĻ�ѧ����ʽΪ �� �������ɷ����Ƿֱ����������²��룺
С�����룺��������Na2CO3
Сǿ���룺��������Ca(OH)2��Na2CO3
����Ϊ˭�IJ����Ǵ���� �������� ���㻹�������IJ����ǣ��������� ��
�����̽����С��ȡһ��������Һ���Թ��У������еμ��˼���ϡ���ᣬ��û�����ݣ��������ó����ۣ�û��Na2CO3��
����ѡ��CO2��������ʣ����һ��ʵ�鷽����֤��IJ��롣
|
ʵ�鲽�� |
������Ӧ���� |
|
|
|
����˼��Сǿ��ΪС���Ľ��۲����ܣ������� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)ij��ȤС�鷢��һ�������ɻ��������ϣ����ϱ��ϵijɷ��������ơ������ʳ�Ρ�����Ҫ̽���������ɻ��������ж�����Щ���ʶ�Ѽ�������á�����ȡ����������ˮ���ܽ⣬��ֽ������ˣ�ȡ��Һ̽����ɷ֡�
����������衿���Ƕ���Ϊ��Һ��һ����NaCl��NaOH�������������ƵĻ�ѧ����ʽΪ �� �������ɷ����Ƿֱ����������²��룺
С�����룺��������Na2CO3
Сǿ���룺��������Ca(OH)2��Na2CO3
����Ϊ˭�IJ����Ǵ���� �������� ���㻹�������IJ����ǣ��������� ��
�����̽����С��ȡһ��������Һ���Թ��У������еμ��˼���ϡ���ᣬ��û�����ݣ��������ó����ۣ�û��Na2CO3��
����ѡ��CO2��������ʣ����һ��ʵ�鷽����֤��IJ��롣
| ʵ�鲽�� | ������Ӧ���� |
|
|
|
����˼��Сǿ��ΪС���Ľ��۲����ܣ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ�鲽�� | ������Ӧ���� |
| | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ̩���н����еڶ�ѧ����Ӧ��ѵ����ѧ�Ծ� ���������� ���ͣ�������
(8��)ij��ȤС���ͬѧΪ����һ��ʯ��ʯ��Ʒ��̼��Ƶ�����������ȡ20g��Ʒ�����ձ�����60gϡ��������μ����ձ���ʹ���ַ�Ӧ(��Ʒ���������ʼȲ�����ˮҲ����ϡ���ᷴӦ)��ʵ�����̼���������(����ʵ�����ݶ��������ձ����������Ҳ�����H2O��HCl�Ļӷ�������X�������衢���ˡ�ϴ�ӡ��������)��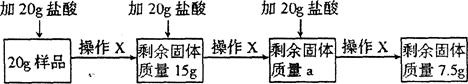
��ش�
(1)CaCO3��Ca��C��������Ϊ ��
(2)ʵ��ʱ�ж��ѳ�ַ�Ӧ�������� ������aΪ g����Ʒ��̼��Ƶ���������Ϊ ��
(3)�����η�Ӧ���˺�������Һ��pH 7(�>������<����=��)��Ϊʹ����Һ
�������ҵõ������ܶ��CaCl2���������ձ��м�������� (�����)
| A��CaO | B��CaCO3 | C��Ca(OH)2 | D��CaCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ɽ��ʡ��̨�г���ѧҵˮƽ���Ի�ѧ�Ծ� ���ͣ�̽����
(8��)ij��ȤС�鷢��һ�������ɻ��������ϣ����ϱ��ϵijɷ��������ơ������ʳ�Ρ�����Ҫ̽���������ɻ��������ж�����Щ���ʶ�Ѽ�������á�����ȡ����������ˮ���ܽ⣬��ֽ������ˣ�ȡ��Һ̽����ɷ֡�
����������衿���Ƕ���Ϊ��Һ��һ����NaCl��NaOH�������������ƵĻ�ѧ����ʽΪ �� �������ɷ����Ƿֱ����������²��룺
С�����룺��������Na2CO3
Сǿ���룺��������Ca(OH)2��Na2CO3
����Ϊ˭�IJ����Ǵ���� �������� ���㻹�������IJ����ǣ��������� ��
�����̽����С��ȡһ��������Һ���Թ��У������еμ��˼���ϡ���ᣬ��û�����ݣ��������ó����ۣ�û��Na2CO3��
����ѡ��CO2��������ʣ����һ��ʵ�鷽����֤��IJ��롣
| ʵ�鲽�� | ������Ӧ���� |
| | |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com