
ij��ѧС��ι��Ƽ�����˸ó���������Ĺ�������ͼ���£���ͼ�ش�
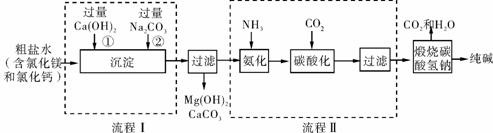
(1)����ͼ�������������ˮ�м���Ca(OH)2��Na2CO3���������˳���ܷ�ߵ���Ϊʲô��
________________________________________________________________________
________________________________________________________________________��
(2)��һ����ѧ����ʽ��ʾ������ͼ���з������ܷ�Ӧ��________________________________________________________________________
________________________________________________________________________��
(3)��������Ȼ����ΪĿ���Ʒ�������백������Ϊ���������Ȼ�泥���ô�������Ƽ�����е�������______________________________________________________________
________________________________________________________________________��
(4)��ҵ��������������У�̼�ữʱ��Һ������̼�����ƶ�û�������Ȼ�淋�ԭ����________________________________________________________________________
________________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ݰ˹֡�֮һ�������������֣����������ֻ������Բ���ɫ��������Ϊī֭�е�̼(����)��
A�����п�ȼ�� B���ڳ����������ȶ�
C������������ D�����л�ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
õ�廨�к�����é�����Ȼ����Ͷ����ͷӵ����ʡ�����㣺
��1�������ͷӣ�C10H12O2������Է�������Ϊ ��
��2�������ͷ���̼���⡢������Ԫ�ص������� ��
��3�������ͷ���̼Ԫ�ص��������� �������ȷ��0.1%����
��4��16.4 g�����ͷ��к� g��Ԫ�أ������ȷ ��0.1 g����
��0.1 g����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵ�ת������ͨ����һ����Ӧʵ�ֵ���(����)��
A��CuO�D��Cu(OH)2 B��KNO3�D��NaNO3
C��Cu�D��CuCl2 D��Na2CO3�D��NaCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ӧ����ϧ�Լ���ѪҺһ����ϧÿһ��ˮ�����й���ˮ��˵��������ȷ����(����)��
A��ˮ������Ԫ�غ���Ԫ����ɵ�
B��������ˮ���뾭����������������ŷ�
C���峺������Ȫˮ�Ǵ�����
D��ˮ��Դ�DZ���ģ�һ��Ҫ��Լ��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ������֮Դ������ش�������ˮ�йص����⣺
(1)���������ˮ�к���������Ϊˮ������______________��
(2)ˮ����Ⱦ����Դ��Ҫ�й�ҵ��Ⱦ��ũҵ��Ⱦ��____________��
(3)��������ˮ���ܽ������ˮ�п죬�÷��ӵ����֪ʶ����ԭ����________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ǹ������ʵ�һ�����ӡ������й�ˮ���ӵ�������ȷ����(����)��
A������ʱˮ���ӵ�������
B������ʱˮ���ӵĻ�ѧ���ʷ����ı�
C����Ӧʱ����ˮ���ӵ�ԭ��������ı�
D�����ˮ��������������˵��ˮ�����ǿɷֵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Թ����Һ����ȣ�Һ���� ��һ�㲻�����Թ��ݻ���_________�������Թ�ʱҪʹ���ԹܼУ������Թܼе�_________����Ҫ��Ĵָ����_________�ϣ�����Ӧ�ô��Թܵ�_________�����ף��̶������Թܿڵ�_________��������ʱ���Թ���б�������______
��һ�㲻�����Թ��ݻ���_________�������Թ�ʱҪʹ���ԹܼУ������Թܼе�_________����Ҫ��Ĵָ����_________�ϣ�����Ӧ�ô��Թܵ�_________�����ף��̶������Թܿڵ�_________��������ʱ���Թ���б�������______ ___����ʹ�Թ�
___����ʹ�Թ� �������ȣ�
�������ȣ� Ȼ����Թ����Һ��IJ�λ���ȣ����Ҳ�ʱ������_________�Թܣ�Ϊ�˱������ˣ�����ʱ�мDz���ʹ�Թܿڶ���_________��
Ȼ����Թ����Һ��IJ�λ���ȣ����Ҳ�ʱ������_________�Թܣ�Ϊ�˱������ˣ�����ʱ�мDz���ʹ�Թܿڶ���_________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com