
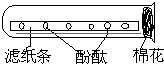
 °“ČēĶ¼ĖłŹ¾ŹµŃ飬½«µĪÓŠ·ÓĢŖŹŌŅŗµÄĀĖÖ½·ÅŹŌ¹ÜÖŠ£¬ŹŌ¹ÜæŚČūÉĻŅ»ĶÅĶŃ֬Ǝ£®
°“ČēĶ¼ĖłŹ¾ŹµŃ飬½«µĪÓŠ·ÓĢŖŹŌŅŗµÄĀĖÖ½·ÅŹŌ¹ÜÖŠ£¬ŹŌ¹ÜæŚČūÉĻŅ»ĶÅĶŃ֬Ǝ£®

 “ŗÓź½ĢÓżĶ¬²½×÷ĪÄĻµĮŠ“š°ø
“ŗÓź½ĢÓżĶ¬²½×÷ĪÄĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
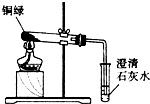
 ×ö²ĖÓƵÄŅ»ÖÖÄŪČā·Ū£¬ĘäÖ÷ŅŖ³É·ÖŹĒĢ¼ĖįĒāÄĘ£®Š”Ć÷·¢ĻÖ½«°čĮĖÄŪČā·ŪµÄČāĄą·Åµ½¹ųÖŠ²¢¼ÓČėŹ³“׵ȵ÷ĮĻÉÕÖóŹ±²śÉśĮĖ“óĮæĘųÅŻ£®Ėū¶Ō“ĖĘÄøŠŠĖȤ£¬¾ö¶Ø¶ŌĘä½ųŠŠĢ½¾æ£®
×ö²ĖÓƵÄŅ»ÖÖÄŪČā·Ū£¬ĘäÖ÷ŅŖ³É·ÖŹĒĢ¼ĖįĒāÄĘ£®Š”Ć÷·¢ĻÖ½«°čĮĖÄŪČā·ŪµÄČāĄą·Åµ½¹ųÖŠ²¢¼ÓČėŹ³“׵ȵ÷ĮĻÉÕÖóŹ±²śÉśĮĖ“óĮæĘųÅŻ£®Ėū¶Ō“ĖĘÄøŠŠĖȤ£¬¾ö¶Ø¶ŌĘä½ųŠŠĢ½¾æ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
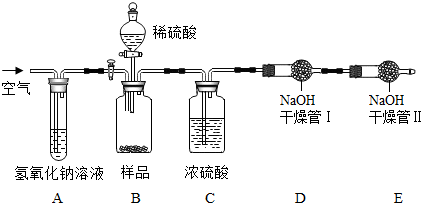
 ŠĖȤŠ”×éĶ¬Ń§ÓĆŅ©Ę·£ŗĻ”ŃĪĖį”¢ŠæĮ£”¢Ģ¼ĖįÄĘ·ŪÄ©¼°ŅĒĘ÷£ŗŅ©³×”¢µĪ¹Ü°“ČēĶ¼ĖłŹ¾ŌŚŹŌ¹ÜÖŠ½ųŠŠŹµŃ飮ŹŌ»Ų“šÓŠ¹ŲĪŹĢā£ŗ
ŠĖȤŠ”×éĶ¬Ń§ÓĆŅ©Ę·£ŗĻ”ŃĪĖį”¢ŠæĮ£”¢Ģ¼ĖįÄĘ·ŪÄ©¼°ŅĒĘ÷£ŗŅ©³×”¢µĪ¹Ü°“ČēĶ¼ĖłŹ¾ŌŚŹŌ¹ÜÖŠ½ųŠŠŹµŃ飮ŹŌ»Ų“šÓŠ¹ŲĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
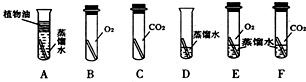
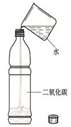 ĪŖĮĖĢ½¾æCO2ÄÜÓėĖ®·“Ó¦£¬Ä³ŠĖȤŠ”×éµÄĶ¬Ń§½ųŠŠĮĖČēĶ¼Ģ½¾æ»ī¶Æ£®ĒėÄć²ĪÓėĖūĆĒµÄŹµŃéĢ½¾æ£®
ĪŖĮĖĢ½¾æCO2ÄÜÓėĖ®·“Ó¦£¬Ä³ŠĖȤŠ”×éµÄĶ¬Ń§½ųŠŠĮĖČēĶ¼Ģ½¾æ»ī¶Æ£®ĒėÄć²ĪÓėĖūĆĒµÄŹµŃéĢ½¾æ£®| ŹµŃé²Ł×÷ | ŹµŃéĻÖĻó | ½įĀŪ |
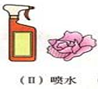 |
Š”»ØČŌĪŖ×ĻÉ« | |
 |
Š”»ØČŌĪŖ×ĻÉ« | |
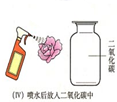 |
Š”»ØÓŠ×ĻÉ«±äŗģÉ« |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com