
某同学为探究Zn、Fe、Cu三种金属的活动性,设计了三组实验:
①将大小相同的Zn、Fe、Cu三种金属片分别插入体积和浓度均相同的稀硫酸中
②将Zn片插入硫酸铜溶液中,Cu片插入硫酸亚铁溶液中
③将Zn片插入硫酸亚铁溶液中,将Fe片插入硫酸铜溶液中
其中可以达到目的是( )
A.①③ B.③ C.①② D.②③
科目:初中化学 来源: 题型:
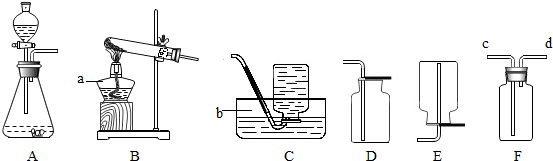
| ||
| ||
查看答案和解析>>
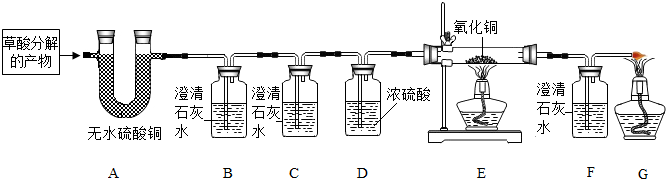
科目:初中化学 来源: 题型:阅读理解
| ||

查看答案和解析>>
科目:初中化学 来源: 题型:阅读理解
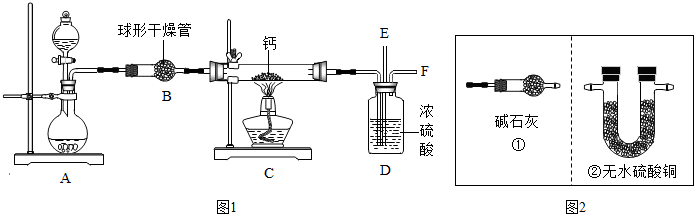
查看答案和解析>>
科目:初中化学 来源:四川省模拟题 题型:实验题
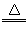 ZnSO4+SO2↑+2H2O计算后,取65.0g锌粒与98%的浓H2SO4(
ZnSO4+SO2↑+2H2O计算后,取65.0g锌粒与98%的浓H2SO4( 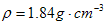 )110mL充分反应锌全部溶解,对于制得的气体,有同学认为可能混有杂质,进行了如下实验探究。查资料:
)110mL充分反应锌全部溶解,对于制得的气体,有同学认为可能混有杂质,进行了如下实验探究。查资料: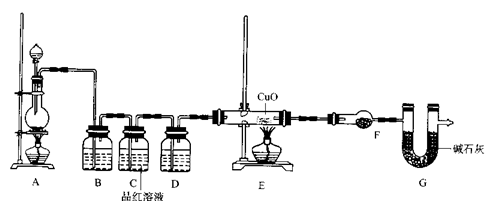
查看答案和解析>>
科目:初中化学 来源:2012年四川省乐山市夹江县中考化学二模试卷(解析版) 题型:填空题
 ZnSO4+SO2↑+2H2O计算后,取65.0g锌粒与98%的浓H2SO4110mL充分反应锌全部溶解,对于制得的气体,有同学认为可能混有杂质,进行了如下实验探究.查资料:(1)SO2气体通入品红溶液会使溶液褪色.(2)随着反应的进行,浓硫酸会变稀.(3)SO2气体的性质与CO2相似.F中盛放的是无水硫酸铜.
ZnSO4+SO2↑+2H2O计算后,取65.0g锌粒与98%的浓H2SO4110mL充分反应锌全部溶解,对于制得的气体,有同学认为可能混有杂质,进行了如下实验探究.查资料:(1)SO2气体通入品红溶液会使溶液褪色.(2)随着反应的进行,浓硫酸会变稀.(3)SO2气体的性质与CO2相似.F中盛放的是无水硫酸铜.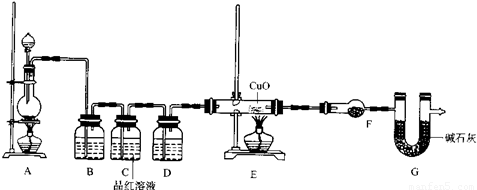
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com