
��������(��ѧʽΪNa2SO3)��Һ�ܺ�BaCl2��Һ��Ӧ�����������ᱵ��ɫ�����������ᱵ�����������У����Ϸ�Ӧ�Ļ�ѧ����ʽ���£�
Na2SO3+BaCl2=BaSO3��+2NaCl��BaSO3+2HCl=BaCl2+SO2��+H2O��ʵ�����е��������������治��������������е�������Ӧ���������ơ�Ϊ�˼��鴢���һƿ���������Ƿ���ʣ�ȡ��������������Ʒ���Ƴ���Һ����ȡ��Һ�����������Ȼ�����Ȼ���ٵ������ᣬ
��1������������δ���ʹ۲쵽�������� ��
��2�����������Ʋ��ֱ��ʣ��۲쵽�������� ��
������ɫ����������ȫ������ϡ���������ɫ������������������ϡ����
���������������������(��ѧʽΪNa2SO3)��Һ�ܺ�BaCl2��Һ��Ӧ�����������ᱵ��ɫ�����������ᱵ�����������в�������������̼������壬�����ᱵ����������ϡ�����ϡ���ᣬ����ȡ��������������Ʒ���Ƴ���Һ����ȡ��Һ�����������Ȼ�����Ȼ���ٵ������ᣬ��1������������δ���ʹ۲쵽�������ǣ�������ɫ����������ȫ������ϡ�����2�����������Ʋ��ֱ��ʣ��۲쵽�������ǣ�������ɫ������������������ϡ���ᡣ
���㣺ҩƷ�Ƿ���ʵ�̽������ѧʵ�鷽�����������


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��3�֣�ʳ����һ������ǿʳƷ��ζ�ij��õ�ζ��������Ҫ�ɷ�Ϊ����(CH3COOH)���������ǵ�����������ء����������ѧ֪ʶ���ش��������⣺
��1��ʳ������ζ����Ϊ�����ˮ��Һ��____ ��������ԡ��������ԡ����ԡ�����
��2�������У�������������Ԫ����____ Ԫ�ء�
��3�������У�����ʳ��������Ǻͼ����ǣ���Ϊ�����������ǵ���Ҫ�ɷַ�Ӧ�����ɴ���ơ�������̼��ˮ���ɴ˿���֪��ǡ���������Ҫ�ɷ��ǣ��ѧʽ��____ ____ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ��ȤС���ͬѧ����ͼ��ʾ��װ�ý�����һ��ȤζСʵ�飨ͼ������̨������������ȥ�����ڢ��м��������������Һ���Լ���������Ƥ�����������ɼУ��۲쵽���������У����ܿ�ð���ݣ���Һ���ְ�ɫ���ǡ��ɴ��ƶϢ��м�������ʿ����ǣ�1�� ��һ��ʱ���ӽ����ɼУ��۲쵽���������У���2�� ����������֪ʶ���رյ��ɼк��в��������ԭ��3�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ������������Һ�������õμӷ�ʽ��Ӧʱ����ҺpH�������Һ����仯�����ߡ�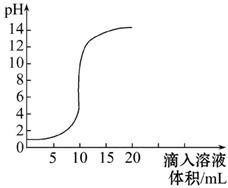
(1)�������ƺ�����ǡ����ȫ��Ӧʱ����Һ��pH 7(����ڡ���С�ڡ����ڡ�)��
(2)���������жϣ��÷�Ӧ�ǽ� (�����������Һ�������ᡱ����ͬ)���� �У������� ��
(3)��������Һ�����Ϊ5 mLʱ��������Һ�е�����Ϊ (д��ѧʽ)�����ڴ���Һ�е���ʯ����Һ����Һ�� ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ҹ�ӵ�зḻ�ĺ�����Դ�����ǿ��ԴӺ�ˮ����ȡʳ�Σ����Դ�Ϊԭ���Ƶþ��й㷺��;���ռ����ȡ����Ҫ�����������£�
(1)���÷紵��ɹ���ԴӺ�ˮ����ȡ���Σ��紵��ɹ����Ҫ������ ��
(2)�Ȼ����ܽ�����н����Ŀ���� ��
(3)д���Ȼ��Ʊ�����Һ��ͨ�������·�����Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ά����C(���Vc����������Ѫ��)��������ˮ���ױ�����������ȱ��Vc�����������ּ�����ˮ�����߲��к��зḻ��Vc��ij�о���ѧϰС�����̽�����£�
̽��һ���ⶨ������Vc�ĺ�����
���������ϡ�Vc�ܺ�����ط�Ӧ��ʹ��ɫ�ĸ��������Һ��ɫ��
����Ʒ������ֱ���ʢ��1 mL��Ũ�ȸ������ϡ��Һ����֧�Թ�����εμӹ�ζ���ϡ�ƻ��֭����֭��0.04%��Vc��Һ���ߵα���ֱ�����������Һ��ɫ��
��ʵ�����ݡ�
| | ��ζ���� | ƻ��֭ | ��֭ | 0.04%��Vc��Һ |
| �μӵĵ��� | 40 | 10 | 20 | 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ���д����������õĻ�ѧ��Դ�������Ȼ�þ���Ȼ��ơ��廯�صȡ��ۺ����ú�ˮ�Ʊ�����þ��������ͼ��ʾ��
(1)������Ҫ�ɷֵĻ�ѧʽ�� ��
(2)����a�������� ����ʵ�����н��д����������Ҫ�IJ����������ձ����������� ��
(3)��ҵұ��þ���õ��MgCl2�ķ�������ӦΪ��
MgCl2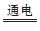 Mg�� ��
Mg�� ��
(4)д���ڢڡ���������Ӧ�Ļ�ѧ����ʽ��
�� ��
�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ϴ�Ӽ�(�������ϴ�·۵�)�������г��õ����ʣ�����Լ��ԡ�С��ȡ���������������ݵķ���ˮ����pH��ֽ���ԵĽ����pH 7(��д������ ����������)���������м�����������ɫ��̪��Һ(��ѧʽΪC20H14O4)������ˮ�� ɫ����̪���� ��Ԫ����ɵģ���̪������̼ԭ�Ӻ���ԭ�ӵĸ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͨ��һ���ѧϰ��Сǿ����ѧ���Ļ�ѧ֪ʶ����������⡣
��1����ʳ��ҵ�����������ƻ������ж�����֪�������Ƶ�ˮ��Һ�Լ��ԣ��Ȼ��Ƶ�ˮ��Һ�����ԣ�Ҫ�����������ƺ��Ȼ��ƿ�ѡ�� ��
��2��θ�����IJ��ˣ��ɷ��ú�����������ҩ�������Სʹ���䷴Ӧ�Ļ�ѧ����ʽΪ ��
��3���·��ϵ����ۣ������ü���ϴ�Ӽ���ˮϴ������ԭ���� ��Ҳ����������ϴ������ԭ���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com