
���� ��1������Ԫ�ص���������=$\frac{Ԫ�ص����ԭ��������ԭ�Ӹ���}{���������Է�������}$��100%���м��㣺
��2�������⻯�ƺ�ˮ��Ӧ�������������ƺ�������Ȼ���������е����ݽ��м��㣻
��3�����������غ㶨�ɽ��з�����
��� �⣺��1��252g�⻯������Ԫ�ص�����Ϊ��252g��$\frac{2��1}{40+2��1}$��100%=12g��
��2������������������Ϊx
CaH2+2H2O�TCa��OH��2+2H2��
42 4
252g x
$\frac{42}{252g}$=$\frac{4}{x}$
x=24g
��3��ͨ��������֪���μӷ�Ӧ��ˮ��Ҳ������Ԫ�أ������⻯������Ԫ�ص�������������������������ȣ���Ϊˮ�е���Ԫ��Ҳ���뷴Ӧ����������
�ʴ�Ϊ����1��12g��
��2��24g��
��3������ȣ���Ϊˮ�е���Ԫ��Ҳ���뷴Ӧ����������
���� ���������û�ѧ����ʽ��ʾ��Ӧ�����ʵ�������ϵ���еĻ������㣬���ʱҪע����㲽�������������������ȷ��


 53������ϵ�д�
53������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼ��ʾ�е�ʵ��������Ǵ���ģ���Ҫ�ش����ʲô�ط�����ָ��������ɵĺ����
��ͼ��ʾ�е�ʵ��������Ǵ���ģ���Ҫ�ش����ʲô�ط�����ָ��������ɵĺ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
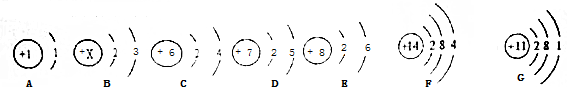
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ľ̿��������ȼ�շ����� | |
| B�� | ��˿��������ȼ�վ��ң��������䣬���ɺ�ɫ���� | |
| C�� | ͭ˿�ڿ����м��ȱ�� | |
| D�� | ������ϡ��������Һ�������ݣ���Һ�����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼ���ܱ���ϵ��ij��Ӧ�Ļչ�ʾ��ͼ����
��ͼ���ܱ���ϵ��ij��Ӧ�Ļչ�ʾ��ͼ���� ���͡�
���͡� ���ֱ��ʾ��ͬԭ�ӣ�
���ֱ��ʾ��ͬԭ�ӣ� B��
B�� C��
C��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | A | B | C | D |
| ��Ӧǰ����/g | 2 | 13.5 | 24 | 6 |
| ��Ӧ������/g | 10.5 | X | 0 | 6 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com