
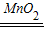 2H2O+O2����
2H2O+O2���� 2H2O+O2����
2H2O+O2����


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
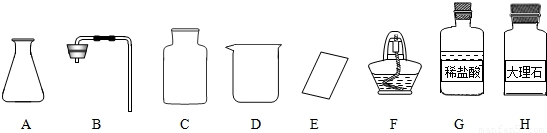
�γ���2013���п���ѧʵ�鿼���У�����������ȡ���ڶ�����̼����ȡ��������50g 5%��NaCl��Һ������������ǩ,��ѧ����ǩȷ��һ��������п��顣
��1����ͬѧ��ǩ������ʵ���ң����ֱ���ʵ��������Ҫ��������������ҩƷ��
��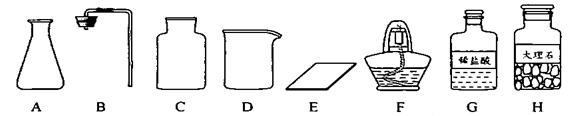 ��ͼ������D�������� ��F�������� ��
��ͼ������D�������� ��F�������� ��
�ڼ�ͬѧ�鵽�Ŀ�ǩӦ���� ������ĸ���ţ���
A����������ȡ B�� ������̼����ȡ
����ȡ������ķ�Ӧԭ��Ϊ ���û�ѧ����ʽ��ʾ������ȡһƿ�����壬Ӧѡ��
�������� ������ĸ���ţ���
�ܼ�ͬѧʵ�����Ҫ����ʾ�����£������������������
���װ�á���������ԡ� ���ռ����塣
�������������巢��װ�û�������ȡ�������壬��д������һ�ַ�Ӧ�Ļ�ѧ����ʽ ��
��2����ͬѧ����ɡ�����50g 5%��NaCl��Һ��ʵ������У�ͨ�����㣬�����NaCl g����ȡˮԼ mL���ܽ�NaClʱ�õ��IJ������������� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
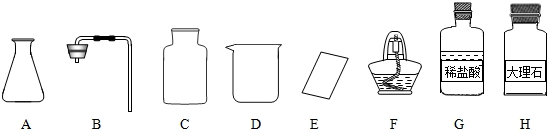
�γ���2013���п���ѧʵ�鿼���У�����������ȡ���ڶ�����̼����ȡ��������50g 5%��NaCl��Һ������������ǩ,��ѧ����ǩȷ��һ��������п��顣
��1����ͬѧ��ǩ������ʵ���ң����ֱ���ʵ��������Ҫ��������������ҩƷ��
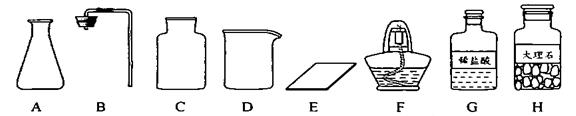
����ͼ������D�������� ��F�������� ��
�ڼ�ͬѧ�鵽�Ŀ�ǩӦ���� ������ĸ���ţ���
A����������ȡ B�� ������̼����ȡ
����ȡ������ķ�Ӧԭ��Ϊ ���û�ѧ����ʽ��ʾ������ȡһƿ�����壬Ӧѡ�õ������� ������ĸ���ţ���
�ܼ�ͬѧʵ�����Ҫ����ʾ�����£������������������
���װ�á���������ԡ� ���ռ����塣
�������������巢��װ�û�������ȡ�������壬��д������һ�ַ�Ӧ�Ļ�ѧ����
ʽ ��
��2����ͬѧ����ɡ�����50g 5%��NaCl��Һ��ʵ������У�ͨ�����㣬�����
NaCl g����ȡˮԼ mL���ܽ�NaClʱ�õ��IJ������������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com