
 ��Һ��������������������ж���ʮ����Ҫ�����ã�
��Һ��������������������ж���ʮ����Ҫ�����ã�| ʵ���� | a | b | c | d | e |
| ����KNO3��������� | 40 | 45 | 50 | 55 | 60 |
| ������Һ���� | 90 | 95 | 100 | 105 | 105 |
| �¶�/ | 0 | 20 | 40 | 60 | 80 |
| �ܽ��/g | 0.18 | 0.16 | 0.14 | 0.11 | 0.09 |
���� ��1��������Һ��Ĺ����Һ�����γɵIJ���һ�����ȶ��Ļ������������Һ��������Һ���Һ���Һ�����γɵIJ���һ�����ȶ��Ļ������������Һ����Һ�Ǿ�һ���ȶ��Ļ�����Һ�ı��������Ǿ�һ�ԡ��ȶ��ԣ����ڻ���
��2��������Һ�������������
��3�����ݱ����е�ʵ�����ݣ������Һ�������ܼ���������������֮�͵Ĺ�ϵ������ܽ�ȣ��������ܼ���ͬ�����������Խ�࣬��������������Խ����н⣻
��4�����ݱ����е�ʵ�������Լ��ܽ�ȵĺ�����н��
��5������������ܽ������ѹǿ���������������ѹǿ�ļ�С����С���н��
��6��������������=��Һ���������ʵ������������ɸ�����Һ�����������ʵ�������������������Һ����Ҫ�����ʵ��������ٸ����ܼ�����=��Һ����-�������������������ˮ��������
��7��������Һϡ��ǰ�����ʵ��������䣬���������з������
��� �⣺��1��A��ʳ��������ˮ���õ���Һ��
B������������ˮ���õ���Һ��
C��ζ��������ˮ���õ���Һ��
D��֥���Ͳ�����ˮ���õ�����Һ��
��ѡD��
��2������ǵ�ľƾ���Һ���ܼ�Ϊ�ƾ�������ƾ���
��3����a��b��c��d��eʵ������������Ϊ50g��4��ˮ�зֱ����40g��45g��50g��55g��60g��KNO3���壬�õ���Һ�����ֱ�Ϊ90g��95g��100g��105g��105g���ɼ�e��δ���ܽ��KNO3���壻���e��
�ڸ��ݱ����е�ʵ������d��e����֪50gˮ������ܽ�55g����أ������ܽ�ȵĺ��壺�ڸ��¶��£�100gˮ������ܽ�110g����أ����Ը��¶���KNO3���ܽ����110g�����110��
��ʵ��a��ʵ��d���Ƶ���Һ�У��ܼ�ˮ��������Ϊ50g���������ܼ���ͬ�����������Խ�࣬��������������Խ����ʵ��a�����ʵ�����Ϊ40g����ʵ��2b�����ʵ�����Ϊ45g����ʵ��c�����ʵ�����Ϊ50g����ʵ��d�����ʵ�����Ϊ55g��ʵ��a��ʵ��d���Ƶ���Һ�����ʵ����������Ĵ�СΪ��a��b��c��d�������������
���ڲ��ı��¶ȵ�����£����ü���ˮ�ķ����������ʵ����������������ˮ������Ϊx��$\frac{55g}{105g+x}$��100%=30%�����x=78.3g���������78.3��ˮ��
��4��A����ͼʾ��֪20��ʱ��A���ܽ��Ϊ0.16g����A��ȷ��
B��A���ʵ��ܽ�����¶ȵ����߶���С������40��ʱ������A�ı�����Һ�¶Ȼ��������ʣ���B��ȷ��
C.60��ʱA���ܽ��Ϊ0.11g������60����100gˮ�м���0.20gA��ֽ��裬���岻����ȫ�ܽ⣬��C��ȷ��
D����ͼʾ��֪A���ܽ�����¶ȵ����߶���С����D����
��ѡ��D��
��5������ʱ�����������ݴӹ���ð����˵������ѹǿ�ļ�С��˵��������ܽ������ѹǿ�ļ�С����С������ܽ�ȣ�
��6��ʵ����������������4%���Ȼ�����Һ50g����Ҫ�����Ȼ��Ƶ�����Ϊ50g��4%=2g���ܼ�����=��Һ����-����������������ˮ������=50g-2g=48g��ˮ���ܶ�Ϊ1g/cm3����48g��48cm3=48mL�����2��48��
��7������Ҫ8%���Ȼ�����Һ������Ϊx��������Һϡ��ǰ�����ʵ��������䣬��x��8%=50g��4% x=25g�����25��
���� ������Ҫ�����ܽ�ȵĶ�������жϳ����¶�������ص��ܽ�ȣ������ܽ�Ⱥ��������������ı�ʾ���������н��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ü�ȩ���ݺ���Ʒ | |
| B�� | ��֬��ʹ�˷��֣���Ҫ����ʳ������ʳƷ | |
| C�� | ���Һõļ����з����ʵ��ļӵ�ʳ�� | |
| D�� | Ϊ�����㳦�������������ӹ������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �����ϵ���˿����������ɻ���ʼ�ɡ�����ش��������⣮
�����ϵ���˿����������ɻ���ʼ�ɡ�����ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
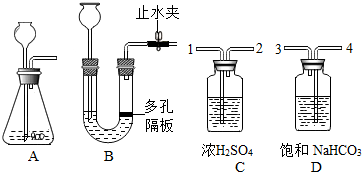 ��ͼ�ش��������⣮
��ͼ�ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
| ����NaOH��Һ������/g | 50.0 | 100.0 | 150.0 | 200.0 | 250.0 |
| ���ɳ���������/g | 0.0 | 2.5 | 8.6 | 9.8 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������ˮ������Һ�����е����� | |
| B�� | ���˺����ɫ����Һ��һ���Ǵ����� | |
| C�� | ��ˮ�ķе��ˮ�͡����̵��ˮ�� | |
| D�� | ���塢Һ������嶼���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com