

���� ��1������һ��������������Һ�IJ��裺���㡢��������ȡ�����ܽ⣮
��2�����ݹ���ҩƷ��ȡ�÷��������ʵ�����=���������+��������������з������
��3��������ƽ��ʹ�÷������������룬���̵������������̵���������������������з������
��4��NaCl������ϷŻ�����ʱ������10g��������ȱ��������ʵ�ʳ�ȡ���Ȼ��Ƶ�����ƫ�٣��ݴ˽��з������
��� �⣺��1������100g������������Ϊ12%��NaCl��Һ�IJ��裺���㡢��������ȡ�����ܽ⡢װƿ��ǩ����ͼʾ����ű�ʾΪ���ۢݢڢܢ٣�
��2����ͼ�еIJ��������ֱ��ǹ��ƿ����Ͳ���ձ��Ͳ�������
��3������NaClʱ����ƽƽ����״̬��ͼ����ʾ��������ʾλ�ü�ͼ�������Ϊ2.4g�����ȡ��NaCl����Ϊ10g+5g+2.4g=17.4g��
��4��NaCl������ϷŻ�����ʱ������10g��������ȱ��������ʵ�ʳ�ȡ���Ȼ��Ƶ�����ƫ�٣�����������Һ����������С��10%��
�ʴ�Ϊ��
��1���ۢݢڢܢ٣���2���������� ��3��17.4g����4��С�ڣ�
���� �����ѶȲ�����ȷ����һ������������������Һʵ�鲽�衢ע����������ȷ�����Ĺؼ���


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
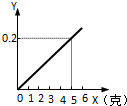 ij������������ϡ���ᷴӦʱ��������ϵ��ͼ��ʾ��x��ʾ�����������ĵ�������Y��ʾ�������������������жϲ��������л���������ǣ�������
ij������������ϡ���ᷴӦʱ��������ϵ��ͼ��ʾ��x��ʾ�����������ĵ�������Y��ʾ�������������������жϲ��������л���������ǣ�������| A�� | Ag | B�� | Zn | C�� | Cu | D�� | Mg |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �������������Ĵ��������������⣮��ͼ��ij�����������������������д������ۺ����õIJ��ֹ������̣�
�������������Ĵ��������������⣮��ͼ��ij�����������������������д������ۺ����õIJ��ֹ������̣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
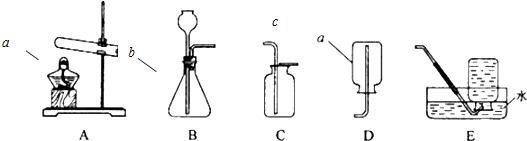
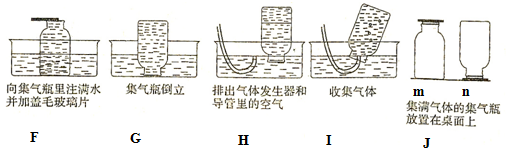
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
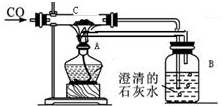 ��λͬѧ���������ʵ�飨װ�ü�����ҩƷ��ͼ��ʾ������ϸ�۲��ش��������⣮
��λͬѧ���������ʵ�飨װ�ü�����ҩƷ��ͼ��ʾ������ϸ�۲��ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ʣ�������ʯī����ͭ | B�� | ������ɱ�����ˮ������ͭ | ||
| C�� | ������ơ���ʯ�ҡ���ˮ | D�� | �л���Ҵ��������ʡ�̼��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com