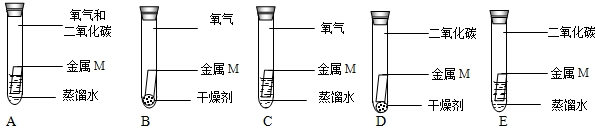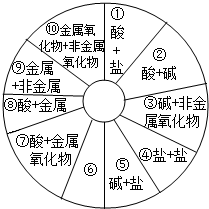
��2011?���죩���ʡ�������ᡢ��Σ���Щ��ͬ��������֮�䷴Ӧʱ�����������Σ�����ͬѧ��������ͼ��ʾ��֪ʶ����ͼ��
��1������дһ�ַ���ͼ����Ϣ�ϵ�Ļ�ѧ����ʽ��Ҫ����Ϣ�����ͼ����������ظ���
����������Һ��Fe+CuSO4=FeSO4+Cu
����������Һ��Fe+CuSO4=FeSO4+Cu
��
��2�������������ͷ�ķ������л������л��ᣬ���仯ѧʽΪHR��R��ʾ�л�����ɷ֣���������ͷǰ����С�մ������õ���ͷ�кܶ�С�ף������������ɿ�������ζ����д��������Ӧ�Ļ�ѧ����ʽ
HR+NaHCO3�TCO2��+H2O+NaR
HR+NaHCO3�TCO2��+H2O+NaR
���÷�Ӧ����Ӧ������ͼ���õ��������
��
��
���Ӣ�-����ѡ������룩��
��3��ͨ�������ͬ�����ʾ������ƵĻ�ѧ���ʣ����磺SO
2��ˮ��Ӧ��CO
2��ˮ��Ӧ���ƣ�SO
2+H
2O=H
2SO
3��H
2SO
3=H
2O+SO
2�������ܽ�����CaSO
3��CaCO
3���ƣ���Ϊ�����Σ���֪úȼ���������������к���SO
2�����壬��ֱ���ŷŵ������У��ײ������꣮����ú�в���������ʯ�ң���ʯ������SO
2��Ӧ���Ӷ�����SO
2������ŷţ���д��SO
2����ʯ�ҷ�Ӧ�Ļ�ѧ����ʽ
SO2+CaO�TCaSO3
SO2+CaO�TCaSO3
��SO
2��ʵ�����Ʒ���CO
2��ʵ�����Ʒ�������������֮������д��H
2SO
4��Na
2SO
3��Ӧ��ȡSO
2�Ļ�ѧ����ʽ
Na2SO3+H2SO4�TNa2SO4+SO2��+H2O
Na2SO3+H2SO4�TNa2SO4+SO2��+H2O
������SO
2ͨ�������ij����ʯ��ˮ�е�������
������ɫ����
������ɫ����
��
��4��Fe
3O
4�ɿ�����Fe
2O
3��FeO��ɣ���Fe
3O
4�Ļ�ѧʽҲ����д��FeO?Fe
2O
3�����Fe
3O
4�����ᷴӦ�Ļ�ѧ����ʽ
Fe3O4+8HCl=2FeCl3+FeCl2+4H2O��FeO?Fe2O3+8HCl=2FeCl3+FeCl2+4H2O
Fe3O4+8HCl=2FeCl3+FeCl2+4H2O��FeO?Fe2O3+8HCl=2FeCl3+FeCl2+4H2O
��





 ��2011?���죩���ʡ�������ᡢ��Σ���Щ��ͬ��������֮�䷴Ӧʱ�����������Σ�����ͬѧ��������ͼ��ʾ��֪ʶ����ͼ��
��2011?���죩���ʡ�������ᡢ��Σ���Щ��ͬ��������֮�䷴Ӧʱ�����������Σ�����ͬѧ��������ͼ��ʾ��֪ʶ����ͼ��