
ij������ͭ��Ʒ�л�������ľ̿�ۣ�Ϊ�ⶨ����Ʒ������ͭ������������ijͬѧ���������װ�ý���ʵ�飨N2���μӷ�Ӧ����������������ʵ���Ӱ�죩��
 �� ��ʵ����Ҫ�ⶨ��������Ӧǰ��װ�â�������� ��9�� ������
�� ��ʵ����Ҫ�ⶨ��������Ӧǰ��װ�â�������� ��9�� ������
�� ��ַ�Ӧ��װ�â������������0.44 g�������ɵĶ�����̼�����ʵ����� ��10�� mol����μӷ�Ӧ���������Ƶ����ʵ�����������ݻ�ѧ����ʽ��ʽ���㣩 ��11��
�� Ϊ�ﵽʵ��Ŀ�ģ��Է�Ӧǰ����������Һ������������Ҫ���� ��12�� g��
�� ���ڸ�ʵ�������˵����ȷ������13�� ��
A������ɫ��ĩȫ����Ϊ������ɫʱ����ֹͣ����
B��ʵ�����ʱ��Ϩ��ƾ���ƻᵼ�¢���Һ�嵹�������
C��ֻ������Ӧǰ��װ�â��й������� Ҳ�ɴﵽʵ��Ŀ��
Ҳ�ɴﵽʵ��Ŀ��
D�����������浪�����ʵ��Ҳ�ɴﵽʵ��Ŀ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
С���Ǹ���ѧϰ��ѧ����ѧ�˻�ѧ��������������֪ʶ��������ȷ����
�ټ�����п��Խ��;�ˮ��Ӳ�ȣ�
��ij���ӵĽṹʾ��ͼΪ ������һ������ԭ�ӣ�
������һ������ԭ�ӣ�
��Ϊ̽��̼����淋����ȶ��ԣ��������е�ȼʵ�飻
�ܽ����ܶ���Ҫ���ƻ����飬��Ϊ������̼���ж��ԣ�
��Ϊ�۲����þ����ɫ����ɰֽ��ĥþ����
��ƿ�ϵĵι�ʹ�ù�������ϴ��ֱ�ӷŻ�ԭƿ��
A���٢ڢ� B���٢ݢ� C���ڢۢ� D���٢ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�̽����������غ����У���ˮ��Դ�ḻ����ˮɹ�ο��Եõ����Σ������к�
����ɳ�Ȳ��������ʣ����ܺ�MgCl2��CaCl2��Na2SO4�ȿ��������ʣ�ijͬѧ���
 ����ʵ�鷽���ᴿ���Σ�ͬʱ��֤�����������Ƿ���ڡ�
����ʵ�鷽���ᴿ���Σ�ͬʱ��֤�����������Ƿ���ڡ�

��ش��������⣺
��1��������������� ��������~���о��õ�һ�ֲ���������������
�ڲ������������� ��
��2����ʵ����Եó����ô�����һ�������Ŀ����������� ��
��3��������������b��һ�����е������� ��
��4�����ᴿ�����У�������ϡ�����Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵ������� ��ѧʽһ�µ���
��ѧʽһ�µ���
A������NaOH B����ʯ�� CaO C���ɱ�H2O D��ʳ��NaCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ɫ������������CH4��H2��CO��CO2��H2O�е�ij����������ɡ�Ϊȷ����ɷ����ν���������ʵ�飨����ÿһ����Ӧ����ȫ������ͨ������ʯ��ˮ����Һ����ǣ���ͨ���������ƹ��壬�������ӣ�����O2�е�ȼ��ȼ�ղ�����ʹ��ɫCuSO4��ĩ��Ϊ��ɫ����ԭ�������ijɷݲ�������
A��CO2��H2O��CH4 B��CO2��H2O��H2
C��CO2��H2O��CO D��CO2��CO��CH4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��H2SO4 ���Ӻ�1��H3PO4�����У�����ȷ����( )
���Ӻ�1��H3PO4�����У�����ȷ����( )
A.������������һ�� B.��ԭ�Ӹ���һ����
C.����Է�������һ�� D.��Ԫ�غ���Ԫ�صĸ���һ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڻ�ѧ������û�������İ���������Ҳ����ʻ������������ڲ�ͬ��λ�ñ�ʾ�Ų�ͬ�ĺ��塣���л�ѧ���������֡�2����ʾ��������ȷ���ǣ� ��
A��Mg2+��һ��þ���Ӵ�2����λ�����  B��CO2��������̼�����к�����ԭ��
B��CO2��������̼�����к�����ԭ��
C��2H��2����Ԫ�� D��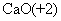 �������ƵĻ��ϼ�Ϊ+2��
�������ƵĻ��ϼ�Ϊ+2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���ǣ�������
�� A.ȼ���������������õĻ�ѧ��Ӧ֮һ
�� B.��ȼ���¶ȴﵽ�Ż�㼴��ȼ��
�� C.ͼ�鵵���������ˮ�������������
�� D.���������˾ƾ��ƣ��ƾ�������ȼ�գ���ˮ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ����������ع�������100.0 g������������Ϊ3.0%���������Һ������˵����ȷ���ǣ� ��
ʵ����������ع�������100.0 g������������Ϊ3.0%���������Һ������˵����ȷ���ǣ� ��
A����50mL��Ͳ��ˮ
B�����������������ƽ�����̳�ȡ
C��������ֱ��Ͷ��ͯͲ���ܽ�
D������õ���Һװ�����б�ǩ����ͼ4�����Լ�ƿ�У�����ƿ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com