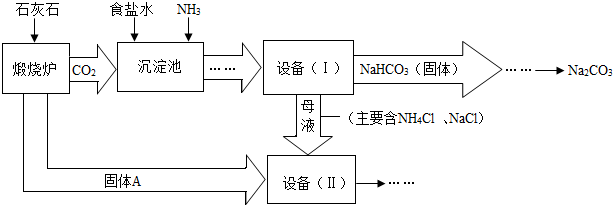
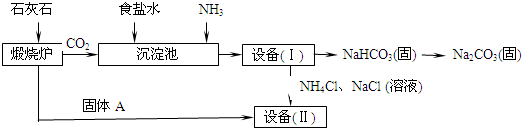
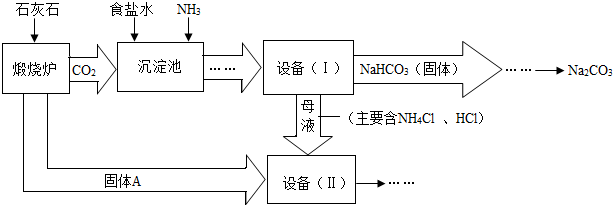
��ҵ�Ͽ�����ʳ�κ�ʯ��ʯΪԭ����ȡ���Na
2CO
3���������Ĺؼ����ڳ��������� NaCl��NH
3��CO
2��H
2O�����ܶ��ת����NaHCO
3������������NH
4Cl����Һ������Ҫ�����������£�
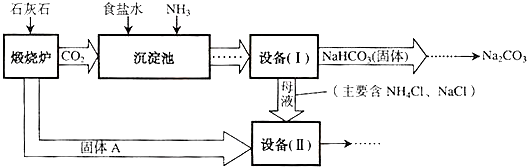
��1��NaHCO
3�׳�
С�մ�
С�մ�
�����������ȷֽ��̼���ƺ����������д����Ӧ�Ļ�ѧ����ʽ
����������������θ����࣬��Ӧ����ʽ��
NaHCO3+HCl�TNaCl+H2O+CO2��
NaHCO3+HCl�TNaCl+H2O+CO2��
��
��2�����ڻ�ѧʵ��������롰�豸��I�����еĻ����õ��IJ���������
����
����
��
��3��д���豸�� II���з����ĸ��ֽⷴӦ�ķ���ʽ
Ca��OH��2+2NH4Cl�T2NH3��+2H2O+CaCl2
Ca��OH��2+2NH4Cl�T2NH3��+2H2O+CaCl2
��
��4���ԡ��豸�����е�ĸҺ������ˮ������ڱ������������õ�������
NaCl��NH3
NaCl��NH3
����д��ѧʽ����
��5��ij��ȤС��ȡ80��ʯ��ʯ��Ʒ��������ʵ�飨�������������չ����в��ֽ⣩����÷�Ӧ��ʣ������������ʱ��Ĺ�ϵ���£�
| ��Ӧʱ�� |
T0 |
T1 |
T2 |
T3 |
T4 |
T5 |
T6 |
| ��Ӧ���������� |
80 |
75 |
70 |
66 |
62 |
58 |
58 |
��ش��������⣺
��ʯ��ʯ��ȫ��Ӧ�����ɶ�����̼������Ϊ
22
22
�ˣ�
�ڸ�ʯ��ʯ��Ʒ��̼��Ƶ�����������д��������̣�


 Na2CO3��CO2����2H2O
Na2CO3��CO2����2H2O ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д� A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�