
���� �����ǵ���Ҫ�ɷ���̼��ƣ�̼�����ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼���������غ㶨�ɣ��ձ������ʼ��ٵ�������Ϊ���ɶ�����̼���������ɷ�Ӧ�Ļ�ѧ����ʽ��ʽ������μӷ�Ӧ��̼��Ƶ����������з������
��� �⣺��1���������غ㶨�ɣ����ɶ�����̼������Ϊ5g+45g-48.9g=1.1g��
��2����μӷ�Ӧ��̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 1.1g
$\frac{100}{44}=\frac{x}{1.1g}$ x=2.5g
�𣺣�1��1.1����2��5g�ü�������̼��Ƶ�����Ϊ2.5g��
���� �����ѶȲ������ո��ݻ�ѧ����ʽ�ļ��㼴����ȷ����⣬����ʱҪע�����Ĺ淶�ԣ�


 ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2KClO3$\frac{\underline{\;MnO_2\;}}{\;}$2KCl+3O2�� | B�� | 3CO+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2 | ||
| C�� | 4Fe+3O2$\frac{\underline{\;��ȼ\;}}{\;}$2Fe2O3 | D�� | Mg+O2$\frac{\underline{\;��ȼ\;}}{\;}$MgO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
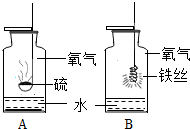 ����A��B��ͼ�ֱ��ʾ��ۡ���˿��������ȼ�յ�ʾ��ͼ��
����A��B��ͼ�ֱ��ʾ��ۡ���˿��������ȼ�յ�ʾ��ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
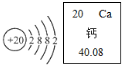 ��Ԫ�ص�ԭ�ӽṹʾ��ͼ����Ԫ�����ڱ�����ʾ����Ϣ��ͼ��ʾ����ش��й����⣺
��Ԫ�ص�ԭ�ӽṹʾ��ͼ����Ԫ�����ڱ�����ʾ����Ϣ��ͼ��ʾ����ش��й����⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
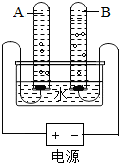 ˮ������֮Դ������ˮ��Դ��ÿ������Ӧ�������Σ���ش������й����⣺
ˮ������֮Դ������ˮ��Դ��ÿ������Ӧ�������Σ���ش������й����⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com