
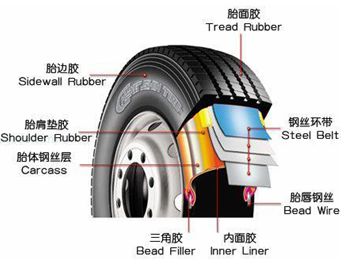
��ͼ��������̥ʾ��ͼ,����ͼ�ش��������⣮
��1����������̥��������ڽ������ϵ��� ר���ǽ����������̥�г���ϡ��������Լ�����̥����ʱ�������,���ٱ�̥�Ļ���,����д��һ��ϡ������Ļ�ѧʽ ��
��2����̥����̥Ȧһ��ʹ�õ��ǺϽ����,û���ô�����,���ܽ�������ԭ����? ��
��3���Ͻ�̥ͼ(�����Ͻ�Ϊ����Ҫ��������)һ�㲻�˽Ӵ���������(������Ϊ��),�����û�ѧ����ʽ��������ԭ�� ��

��1����˿��He(Ne��) ��2���Ͻ�Ӳ�Ƚϴ�Ͻ����ʴ����3��2Al+6HCl=2AlCl3+3H2��
��������
�����������1����������̥��������ڽ������ϵ��Ǹ�˿��ר���ǽ����������̥�г���ϡ��������Լ�����̥����ʱ�������,���ٱ�̥�Ļ���,ϡ������Ļ�ѧʽΪHe����2����̥����̥Ȧһ��ʹ�õ��ǺϽ����,û���ô�����,ԭ���ǺϽ�Ӳ�Ƚϴ�3���Ͻ�̥ͼ(�����Ͻ�Ϊ����Ҫ��������)һ�㲻�˽Ӵ���������(������Ϊ��),�û�ѧ����ʽ��������ԭ��Ϊ2Al+6HCl=2AlCl3+3H2��
���㣺�����IJ��ϡ������Ļ�ѧ����


 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 22����ͼ��������̥ʾ��ͼ������ͼ�ش��������⣮
22����ͼ��������̥ʾ��ͼ������ͼ�ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
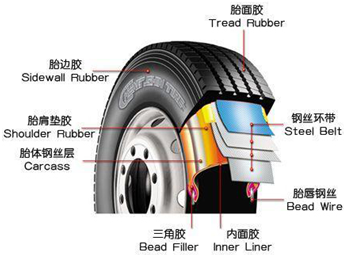
 ����2011��3?15���ᣬ�ع��˽�����̥ԭ�ϲ������⣺�Է���������ԭƬ��������Ӱ����̥���������������˽�����̥�ij���������ȫ��������ͼ��������̥ʾ��ͼ����ش��������⣺
����2011��3?15���ᣬ�ع��˽�����̥ԭ�ϲ������⣺�Է���������ԭƬ��������Ӱ����̥���������������˽�����̥�ij���������ȫ��������ͼ��������̥ʾ��ͼ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר���� ���ͣ������
�鿴�𰸺ͽ���>>
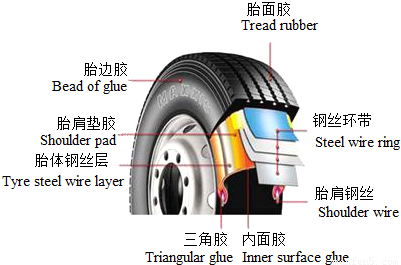
��Ŀ�����л�ѧ ��Դ��2010�갲��ʡ�����С���У�������п���ѧģ���Ծ��������������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com