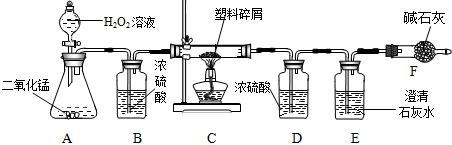
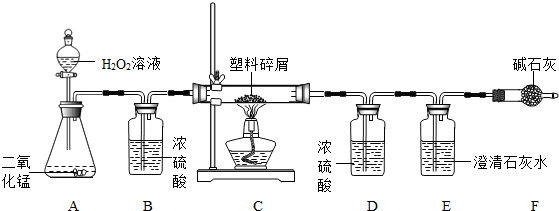
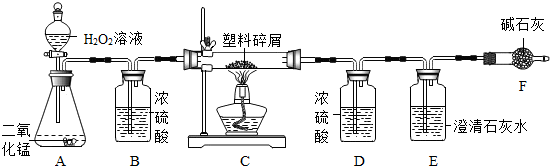
ÓÉÓŚ“óĮæŹ¹ÓĆŅ»“ĪŠŌĖÜĮĻ·½±ć“ü¶ųŌģ³ÉµÄ”°°×É«ĪŪČ¾”±ŅŃ³ÉĪŖŅ»øöŃĻÖŲµÄÉē»įĪŹĢā£®Ä³»Æѧъ¾æŠ”×éµÄĶ¬Ń§¶ŌijÖÖĖÜĮĻ“üµÄ×é³É½ųŠŠ·ÖĪöŃŠ¾æ£Ø׏ĮĻĻŌŹ¾øĆĖÜĮĻÖ»ŗ¬C”¢HĮ½ÖÖŌŖĖŲ£©£®ĖūĆĒÉč¼ĘĮĖČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ£¬Ź¹øĆĖÜĮĻŹŌŃłŌŚ“æŃõÖŠĶźČ«Č¼ÉÕ£¬¹Ū²ģŹµŃéĻÖĻ󔢷ÖĪöÓŠ¹ŲŹż¾Ż”¢ĶĘĖćŌŖĖŲŗ¬Į森£Ø¼īŹÆ»ŅæÉĪüŹÕĖ®ŗĶ¶žŃõ»ÆĢ¼£©

£Ø1£©ŹµŃé×°ÖĆÖŠÓŠŅ»“¦Ć÷ĻŌ“ķĪó£¬ĒėŠ“³öøÄÕż·½·Ø
×°ÖĆBÖŠĒ°Ćęµ¼Ęų¹ÜÓ¦²åČėŅŗĆęĻĀ£¬ŗóĆęµ¼Ęų¹ÜÓ¦øÕŗĆĶø¹żĘæČū
×°ÖĆBÖŠĒ°Ćęµ¼Ęų¹ÜÓ¦²åČėŅŗĆęĻĀ£¬ŗóĆęµ¼Ęų¹ÜÓ¦øÕŗĆĶø¹żĘæČū
£®
£Ø2£©×°ÖĆAÖŠ·ÖŅŗĀ©¶·Ņ²æÉÓĆ³¤¾±Ā©¶·“śĢę£¬³¤¾±Ā©¶·Ó¦øĆ
ÉģČėŅŗĆęŅŌĻĀ
ÉģČėŅŗĆęŅŌĻĀ
£¬×÷ÓĆŹĒ
ŠĪ³ÉŅŗ·ā£¬·ĄÖ¹ĘųĢåŅŻ³ö
ŠĪ³ÉŅŗ·ā£¬·ĄÖ¹ĘųĢåŅŻ³ö
£Ø3£©×°ÖĆEÖŠµÄĻÖĻóŹĒ
³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē
³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē
£¬×°ÖĆFµÄ×÷ÓĆŹĒ
ĪüŹÕæÕĘųÖŠµÄĘųĢ壬·ĄÖ¹½ųČėE×°ÖĆ
ĪüŹÕæÕĘųÖŠµÄĘųĢ壬·ĄÖ¹½ųČėE×°ÖĆ
£Ø4£©×°ÖĆAÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
£®
£Ø5£©Čō×°ÖĆCµÄ²£Į§¹ÜÖŠ·ÅČėµÄĖÜĮĻŹŌŃłÖŹĮæĪŖ5.9g£¬ĖÜĮĻŹŌŃł³ä·ÖČ¼ÉÕŗó£¬×°ÖĆDŌöÖŲ7.2g£¬ŌņøĆĖÜĮĻŹŌŃłÖŠŗ¬ĒāŌŖĖŲµÄÖŹĮæĪŖ
0.8
0.8
g£»
£Ø6£©Čō×°ÖĆ֊ƻӊĮ¬½Ó×°ÖĆB£¬½«Ź¹øĆĖÜĮĻŹŌŃłÖŠĒāŌŖĖŲµÄÖŹĮæ²āĖć½į¹ū
Ę«“ó
Ę«“ó
£Ø Ģī”°Ę«Š””±”¢”°Ę«“ó”±»ņ”°ĪŽÓ°Ļģ”±£©




 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø