
���� ��1�����ݸ���������������õ�������
��2����ʵ��Ϊ����ʵ�飬��ȷ�Ե�Ҫ��ϸߣ��ڼ�����ȴʱ��Ϊ�˷�ֹ����ͭ��ˮ��Ӧ������ͭ���ڸ���������ȴ���Ӷ��õ����������������ͭ��
��3���ڶ���ʵ���У���������һЩ��������ظ����籾ʵ���еĢۼ��ȡ�����ȴ���ݳ����IJ�������Ҫ�ظ�ʹ�ã�ֱ���������γ��������������0.1gΪֹ������Ϊ��ȷ����Ʒ�нᾧˮ�Ƿ��Ѿ���ȫ��ȥ����֤ʵ���ȷ�ԣ�
��4�����ݷ�Ӧǰ������ͭ�����������ʧȥ�ᾧˮ����������������ͭ�����нᾧˮ������
��5��A�����Ⱥ�����δ�������������ȴ������ͭ������ˮ�֣����²��ʧȥ�ĽᾧˮƫС���з�����
B��������Ʒ�к��м����ӷ������ʣ�ʹ����������ƫ���ʧȥ�Ľᾧˮƫ����з�����
C��ʵ��ǰ������Ʒ���в���ʧˮ�����²��ʧȥ�ĽᾧˮƫС���з�����
D������ǰ���õ�����δ��ȫ������Ⱥ�ˮ�ӷ�������ˮ�IJⶨ���ƫ����з�����
��� �⣺��1������ʵ��ʱ��A��������ƽ�����г��������� B���в���������ĥ�� C���ԹܼУ�����Һ�����֧�֣������ȵ��ǹ��壬ҩ�ײ��ã� D���ƾ��ƣ����ȣ� E��������������������ʵ�鲻���������� F�������������Խ���ת�Ƶ�ʹ�ã� G�����������м��ȣ� H��������������ȴʱʹ�ã���ֹ������I�������� �� K�����ż����ڹ̶�������J��ʯ���������ã���������ֱ�Ӽ��ȣ� L��ҩ�ף�����ʱʹ�ã� M������ǯ���г�������
���CEJ��
��2����ʵ��Ϊ����ʵ�飬��ȷ�Ե�Ҫ��ϸߣ��ڼ�����ȴʱ��Ϊ�˷�ֹ����ͭ��ˮ��Ӧ������ͭ���ڸ���������ȴ���Ӷ��õ����������������ͭ������ܣ���ֹ�������տ����е�ˮ������
��3���ڶ���ʵ���У���������һЩ��������ظ����籾ʵ���еĢۼ��ȡ�����ȴ���ݳ����IJ�������Ҫ�ظ�ʹ�ã�ֱ���������γ��������������0.1gΪֹ������Ϊ��ȷ����Ʒ�нᾧˮ�Ƿ��Ѿ���ȫ��ȥ����֤ʵ���ȷ�ԣ�����ȴ�����ڸ������н��У����������������Ʒ�еĽᾧˮ�Ƿ���ȫ��ȥ��
��4��ʹ����ͭ������ȫʧȥ�ᾧˮ ��4��ͨ��������֪������ͭ����������ǣ�W2-W1��ʧȥ�Ľᾧˮ�����ǣ�W3-W2����������ͭ�����нᾧˮ�����ǣ�$\frac{W2-W3}{W2-W1}$��100%�����$\frac{W2-W3}{W2-W1}$��100%��
��5��A�����Ⱥ�����δ�������������ȴ������ͭ������ˮ�֣����²��ʧȥ�ĽᾧˮƫС��
B��������Ʒ�к��м����ӷ������ʣ�ʹ����������ƫ���ʧȥ�Ľᾧˮƫ��
C��ʵ��ǰ������Ʒ���в���ʧˮ�����²��ʧȥ�ĽᾧˮƫС��
D������ǰ���õ�����δ��ȫ������Ⱥ�ˮ�ӷ�������ˮ�IJⶨ���ƫ��
��ѡ��AC��
���� ������Ҫ����������ͭ�����нᾧˮ�IJⶨ���ѶȽϴ���ʵ�����ԭ����ʵ��ע�������ǽ���Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
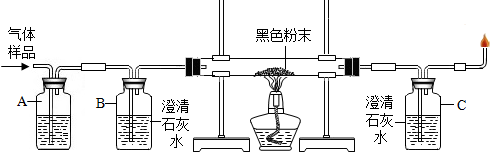
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������
������ ����ʾ��������ͬ��ԭ�ӣ�
����ʾ��������ͬ��ԭ�ӣ�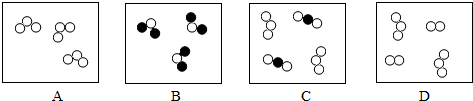
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ�����ʺ��ܼ���ɣ�һ���Ǿ�һ���ȶ���Һ�� | |
| B�� | ��������������ʱ���������ܽ������ѹǿ�����߶���С | |
| C�� | ������Һת��Ϊ��������Һ�����ʵ���������һ����С | |
| D�� | ���¶Ȳ���ʱ��������ʯ��ˮ�м������ƣ���Һ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �٢� | C�� | �ڢ� | D�� | �ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
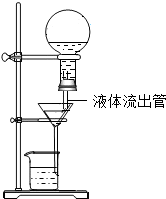 ijѧ��Ϊ��ʹ���˲������Զ�����Һ�壬����ˡ��Զ���Һ��������������ͼ��ʾ���ڵ��õ���ƿ��ʢ�Ŵ����˵�Һ�壬Һ��ӡ�Һ�������ܡ�����©����ΪʹҺ��˳�����£�������롰��������ܡ��������ͨ��
ijѧ��Ϊ��ʹ���˲������Զ�����Һ�壬����ˡ��Զ���Һ��������������ͼ��ʾ���ڵ��õ���ƿ��ʢ�Ŵ����˵�Һ�壬Һ��ӡ�Һ�������ܡ�����©����ΪʹҺ��˳�����£�������롰��������ܡ��������ͨ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com