
���� ��1�����ݵ�̼���þ��Ǽ��ٶ�����̼���ŷŷ������ɣ�
��2����������Դ���ŵ�������ɣ�
��3��������������������з������ɣ�
��4�����õƻ�ʵ����������������ĺ�����
��� �⣺��1������̼���á��������ʩ�ܶ࣬�磺ʹ��˫��ֽ�ţ���̫������ˮ������ú¯��ˮ�����û���һ���Բ;ߣ���ʡʹ����Ʒ����Ʒ�������ã��ᳫ����������ͨ���ߡ������г����еȳ��з�ʽ�ȣ�
��2����ҵ����ȡ������ͨ�����ˮ�õ��ģ���������ˮ��Դ�ḻ�����Դ�ˮ����ȡ������˵����Դ�㷺��������ȼ��ֵ�ߣ���Ϊ����ȼ�ղ�����ˮ������Ⱦ������
��3�����ڿ�������ʴ��ʵ���������������е�������ˮ�����ʼ�����÷�����һϵ�и��ӵĻ�ѧ��Ӧ��Ϊ�˷�ֹ��������ʴ�����dz������������ˢ�ᡢͿ�ͻ�������������������ȸ��DZ���Ĥ�ķ�������Щ�������ܹ���ֹ��ʴ�Ĺ�ͬԭ�Ǹ���������ˮ��
��4�������������������͵�ʱ��ȼ��ȼ�ջ������Ϩ�𣬹ʿ��õƻ�ʵ����������������ĺ�����
�ʴ�Ϊ����1��ʹ��˫��ֽ�ţ���̫������ˮ������ú¯��ˮ�����û���һ���Բ;ߣ���ʡʹ����Ʒ����Ʒ�������ã��ᳫ����������ͨ���ߡ������г����еȳ��з�ʽ�ȣ���2��ԭ����Դ�㡢ȼ��ʱ�ų���ֵ�ߡ������ﲻ����Ⱦ��������3��ˢ�ᡢͿ�͡���ơ����������ֽ�������Ľྻ�����4���õƻ�ʵ����������������ĺ�����
���� ���⿼���֪ʶ��϶࣬�ۺ��Խ�ǿ��Ҫע��ƽʱ֪ʶ�Ļ��ۣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
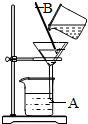 ��ͼ��ʵ�����й��˲���ʾ��ͼ���ش��������⣺
��ͼ��ʵ�����й��˲���ʾ��ͼ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������Һ | B�� | п�� | C�� | ̼������Һ | D�� | ��ɫʯ����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
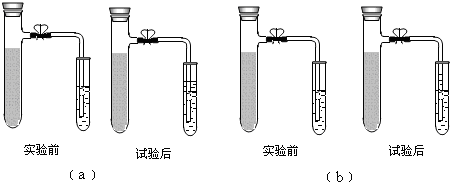

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
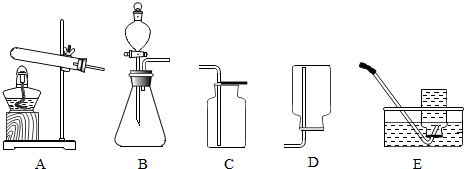
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 2012��3�µף��������سǹܾ�ִ����ԱѲ���в������һ���衰���켦�������Ӷ��ҿ������ڡ����쵰���ϵ�һ����ɴ�����켦������Ҫԭ���ǡ��������ơ��Ȼ��ơ�Ӳ֬�ᡢʳ��ɫ�ء�ʳ��ʯ����ˮ�������ơ����������䷽������Ӳ֬�ữѧʽΪ C18H36O2��
2012��3�µף��������سǹܾ�ִ����ԱѲ���в������һ���衰���켦�������Ӷ��ҿ������ڡ����쵰���ϵ�һ����ɴ�����켦������Ҫԭ���ǡ��������ơ��Ȼ��ơ�Ӳ֬�ᡢʳ��ɫ�ء�ʳ��ʯ����ˮ�������ơ����������䷽������Ӳ֬�ữѧʽΪ C18H36O2���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com