
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
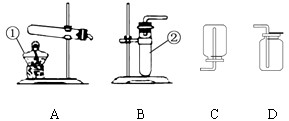
��20�֣�����5�£����и�У�����˻�ѧʵ��������飬һЩͬѧ����ͼ��ʾ��������װ�ý��г����������ȡ������ʵ�顣��ش��������⣺��������ʵ��װ��ͼ�ش�
��1��д��ͼ�б��б�����������ƣ�
a ____________�� b _______________��
��2��С��ͬѧ��ʯ��ʯ��ϡ������ȡ���ռ�һƿ������̼��Ӧѡ��ͼ���е�________��_________װ�ã���װ�ö�Ӧ����ĸ�����÷�Ӧ�ķ��ű���ʽ�� ________________ �ռ�������̼��С�콫ȼ�ŵ�ľ�����ڼ���ƿ�ڣ��������ռ������ɹ۲쵽��������_______________________________��
��3��A��B����װ�þ�������ʵ������ȡ������������ ��ѡ��A��B��װ�ã����������ķ��ű���ʽΪ ��ѡװ��E�ռ�����,���ռ������Ǹ������� �����ʶ�ȷ���ġ�
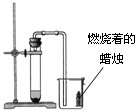
��4��ʵ�������������ƹ�����Ȼ�粒��������ȡ�����������д̼�����ζ��������ˮ���ܶȱȿ���С����ѡ��ͼ�е� �� װ������ȡ�ռ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com