
| ��Ӧʱ�� | t0 | t1 | t2 | t3 | t4 |
| �ձ���ҩƷ����/g | 25.7 | 25.6 | 25.5 | 25.5 | m |
���� ��1������ˮ������ͬʱ�Ӵ�ʱ�������⣻
��2���ᡢ����Һ�ܹ��ٽ�����Ʒ���⣻
��3�����ݱ������ݿ����ж�m��ֵ��
����ϡ���ᷴӦ�����Ȼ���������������Ӧǰ��������Ϊ��Ӧ���������������������������������Լ�������������
���������£���������ľ̿��Ӧ�������Ͷ�����̼��������̼�ܹ�������������Һ���գ�����������Һ���ӵ�������Ϊ��Ӧ���ɶ�����̼�����������ݶ�����̼������
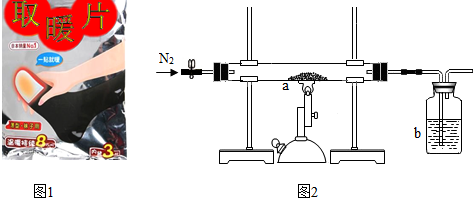
���Լ�������������������һ�����Լ����ѱ��ʵġ�ȡůƬ����Ʒ��Fe2O3������������
��� �⣺��1�����ȼ���Ӵ��������Żᷢ�ȣ�ԭ������Ҫ��������ˮ��ͬ���òŻ����⣮
���������ˮ��
��2����ȡůƬ���е��Ȼ��������Ǽӿ������⣮
����ڣ�
��3�����ɱ����ṩ�����ݿ�֪��t3ʱ�ձ���ҩƷ�������ټ�С��˵����Ӧ�Ѿ����г��ף���˱�����m��ֵΪ25.5��
���25.5��
���衰ȡůƬ����Ʒ�����۵�����Ϊx��
����ϡ���ᷴӦ��������������Ϊ��5.7g+10.0g+10.0g-25.5g=0.2g��
Fe+2HCl�TFeCl2+H2����
56 2
x 0.2g
$\frac{56}{x}$=$\frac{2}{0.2g}$��
x=5.6g��
���5.6��
��ʵ���м���ǰҪ����ͨ��һ��ʱ�䵪�����������ų�װ���еĿ�����
ֹͣ���Ⱥ�Ҫ����ͨ��һ��ʱ�䵪��������Ӧ���ɵĶ�����̼���ܱ�bװ����ȫ���գ��Ӷ����²ⶨ�Ľ��ƫС��
����ų�װ���еĿ�����ƫС��
�ڳ�ַ�Ӧ����ͬѧ�ⶨb�е��Լ�������3.3g��˵����Ӧ������3.3g������̼��
�Լ�������������Һ��������Ӧ�Ļ�ѧ����ʽΪ��2NaOH+CO2�TNa2CO3+H2O��
����������ƣ�2NaOH+CO2�TNa2CO3+H2O��
����������������Ϊy��
2Fe2O3+3C $\frac{\underline{\;\;��\;\;}}{\;}$4Fe+3CO2����
320 132
y 3.3g
$\frac{320}{y}$=$\frac{132}{3.3g}$��
y=8g��
��Ʒ��Fe2O3����������Ϊ��$\frac{8g}{10g}$��100%=80%��
����Ʒ��Fe2O3����������Ϊ80%��
���� ������Ҫ����ѧ�����ü��跨�ͻ�ѧ����ʽ���м�����ƶϵ�������ͬʱ�����˷����������ݵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�


 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ��ˮ�д�Լ��1.67��1021��ˮ���ӣ�˵��ˮ���Ӻ�С | |
| B�� | ǽ�ڿ���ǽ����ŵ����㣬˵�������ڲ����˶� | |
| C�� | 6000L�����ڼ�ѹ������¿�װ���ݻ�Ϊ40L�ĸ�ƿ�У�˵����������Ŀ������ | |
| D�� | һ����̼�Ͷ�����̼���в�ͬ�Ļ�ѧ���ʣ�˵�����ǵķ��ӹ��ɲ�ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���뻯����һ�㲻����������������Ь�� | |
| B�� | ҹ������Һ����й©�����رշ��Ų����� | |
| C�� | ��ʳƷ���г��뵪�����Ա���ʳƷ | |
| D�� | ��ͥ�ô׳�ʱ����������Գ�ȥ������ˮ������CaCO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ɫ�����Һ�� | B�� | 0��ʱ����ɱ� | ||
| C�� | ͨ���£���ֽ⣬���ȶ� | D�� | 100��ʱ��������ˮ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | O2������MgO���� | B�� | O2������MgO���� | C�� | O2����=MgO���� | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ԫ��ԭ�ӵ�������Ϊ39 | B�� | ��Ԫ�ص�������Ϊ51 | ||
| C�� | ��Ԫ�غ��������Ϊ39 | D�� | ��Ԫ�غ˵����Ϊ90 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com