
£Ø9·Ö£©ŹµŃéŹŅÓŠŅ»ĘæĮņĖįČÜŅŗ£¬ĄĻŹ¦ĒėŠ”ŗģĶ¬Ń§Éč¼Ę·½°ø²ā¶ØøĆ·ĻŅŗÖŠĮņĖįµÄÖŹĮæ·ÖŹż”£Š”ŗģĶ¬Ń§ĻČČ”Ņ»½ą¾»Š”ÉÕ±£¬³ĘĘäÖŹĮæĪŖ18.2g£¬Č»ŗóĶłĘäÖŠµ¹ČėÉŁĮæĮņĖį·ĻŅŗŗó³ĘĮ棬×ÜÖŹĮæĪŖ33.2g£¬Ö®ŗ󣬽«Ņ»Ć¶ÖŹĮæĪŖ10.8gµÄĢś¶¤£ØŅŃÓĆÉ°Ö½“ņÄ„Č„ÄźĢśŠā£©·ÅČėøĆŠ”ÉÕ±ÖŠ·“Ó¦£¬“żĢś¶¤±ķĆę²»ŌŁÓŠĘųÅŻ²śÉśŗó£¬ŌŁ“Ī³ĘĮ棬×ÜÖŹĮæĪŖ43.9 g”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©·“Ó¦ÖŠ²śÉśµÄĘųĢåµÄÖŹĮæŹĒ ”£
£Ø2£©¼ĘĖćøĆ·ĻŅŗÖŠĮņĖįµÄÖŹĮæ·ÖŹż£ØŠ“³ö¼ĘĖć¹ż³Ģ£¬¼ĘĖć½į¹ū±£ĮōŅ»Ī»Š”Źż£©”££Ø6·Ö£©
£Ø3£©Čē¹ūĢś¶¤µÄĢśŠāĪ“³ż¾»£¬¶Ō¼ĘĖć½į¹ūµÄÓ°ĻģŹĒ (Ń”Ģī”°Ę«“ó”±”¢”°Ę«Š””±”¢”°ĪŽÓ°Ļģ”±)£¬ŌŅņŹĒ ”£
£Ø1£©0.1g £Ø2£©32.7% £Ø3£©Ę«Š””¢ĮņĖįÓėĢśŠā·“Ó¦
½āĪöŹŌĢā·ÖĪö£ŗ¢Åøł¾ŻÖŹĮæŹŲŗć¶ØĀÉ£¬Éś³ÉĒāĘųµÄÖŹĮæĪŖ£ŗ33.2g+10.8g-43.9g=" 0.1g"
(2)(6·Ö) ½ā£ŗÉč·ĻŅŗÖŠH2SO4ÖŹĮæĪŖx
Fe + H2SO4 £½ FeSO4 + H2”ü
98 2
X 0.1g
98/X=2/0.1g
x="4.9" g
·ĻŅŗÖŠĮņĖįµÄÖŹĮæ·ÖŹż £½4.9g/(33.2g-18.2g)*100% £½32.7£„
¢ĒČē¹ūĢś¶¤µÄĢśŠāĪ“³ż¾»£¬¶Ō¼ĘĖć½į¹ūµÄÓ°ĻģŹĒĘ«Š”,ŅņĪŖĮņĖįÓėĢśŠā·“Ó¦£¬»įŌģ³Éøł¾ŻĒāĘų¼ĘĖ涹ŠčĮņĖįµÄÖŹĮæĘ«ÉŁ£¬¼Ģ¶ųŌģ³É·ĻŅŗÖŠĮņĖįÖŹĮæ·ÖŹżµÄ¼ĘĖćĘ«Š””£
æ¼µć£ŗÖŹĮæŹŲŗć¶ØĀÉ”¢ÓŠ¹Ų»Æѧ·½³ĢŹ½µÄ¼ĘĖć”¢ČÜÖŹÖŹĮæ·ÖŹżµÄ¼ĘĖć


 ĄčĆ÷ĪÄ»Æŗ®¼Ł×÷ŅµĻµĮŠ“š°ø
ĄčĆ÷ĪÄ»Æŗ®¼Ł×÷ŅµĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
£Ø11·Ö£©Ćŗ”¢ŹÆÓĶŗĶĢģČ»ĘųµČ»ÆŹÆČ¼ĮĻŹĒÄæĒ°ČĖĄąŹ¹ÓƵÄ×īÖ÷ŅŖČ¼ĮĻ£¬Ņ²ŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ”£
£Ø1£©ŹÆÓĶÖŠÖ÷ŅŖŗ¬ÓŠ Į½ÖÖŌŖĖŲ£ØĢīŠ“ŌŖĖŲ·ūŗÅ£©£»ĢģČ»ĘųµÄÖ÷ŅŖ³É·ÖŹĒ £ØĢīŠ“»ÆѧŹ½£©”£
£Ø2£©»ÆŹÆČ¼ĮĻŹōÓŚ £ØŃ”Ģī”°æÉŌŁÉś”±»ņ”°²»æÉŌŁÉś”±£©ÄÜŌ“£¬ĘäČ¼ÉÕÅŷŵēóĮ涞Ńõ»ÆĢ¼»įŅżĘšČ«ĒņĘųŗņ±äÅÆ”£Ņ»ÖÖŠĀµÄ“¦Ąķ·½·ØŹĒ½«¶žŃõ»ÆĢ¼ĘųĢåĶØČėŗ¬ÓŠ³¤ŹÆ£ØµŲæĒÖŠ×ī³£¼ūµÄæóŹÆ£¬ŗ¬Įæøß“ļ60%)³É·ÖµÄĖ®ČÜŅŗĄļ£¬ĘäÖŠŅ»ÖÖ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ KAlSi3O8+CO2+2H2O==KHCO3+X+3SiO2”ż£¬ŌņXµÄ»ÆѧŹ½ĪŖ ”£
£Ø3£©¹¤ŅµÉĻ£¬ĆŗĢæČ¼ÉÕĒ°½ųŠŠ·ŪĖéµÄÄæµÄŹĒ ”£Ćŗøō¾ųæÕĘų¼ÓĒæČȵƵ½µÄ½¹Ģ棬ŹĒŅ±ĢśµÄÖŲŅŖŌĮĻ”£ĪŖĮĖ²ā¶Øij³ąĢśæóÖŠŃõ»ÆĢśµÄÖŹĮæ·ÖŹż£¬»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§Éč¼ĘĮĖĮ½ÖÖŹµŃé·½°ø£Ø¼ŁÉčøĆ³ąĢśæóÖŠµÄŌÓÖŹ¼Č²»ČÜÓŚĖ®£¬Ņ²²»·¢Éś·“Ó¦£©”£
·½°øI Č”8£®00g³ąĢśæó·Ū£¬¼ÓČė×ćĮæĻ”ĮņĖį£¬ĶźČ«·“Ó¦ŗó¹żĀĖ£¬µĆµ½1£®60gĀĖŌü”£Ōņ³ąĢśæó·ŪÖŠŃõ»ÆĢśµÄÖŹĮæ·ÖŹżĪŖ ”£
·½°ø¢ņ ČēĶ¼ĖłŹ¾£¬Č”8£®00g³ąĢśæó·ŪÓė¹żĮæµÄ½¹Ģæ·Ū»ģŗĻŗó¼ÓĒæČČ£¬³ä·Ö·“Ó¦”£²āµĆĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦Ē°ŗóÖŹĮæŌö¼ÓĮĖ1£®32g”£Čē¹ū²śÉśµÄ¶žŃõ»ÆĢ¼±»ĒāŃõ»ÆÄĘČÜŅŗĶźČ«ĪüŹÕ£¬øł¾ŻĖłŃ§·“Ó¦3C+2Fe2O3”÷4Fe+3CO2”ü¼ĘĖć£¬³ąĢśæó·ŪÖŠŃõ»ÆĢśµÄÖŹĮæ·ÖŹżĪŖ ”£
[ŹµŃé·“Ė¼]·½°ø¢ņÖŠÓ²ÖŹ²£Į§¹ÜÄŚŌÓŠµÄæÕĘų¶ŌŹµŃé½į¹ūÓŠÓ°Ļģ£¬Õā»įµ¼ÖĀ²ā³öµÄŃõ»ÆĢśµÄÖŹĮæ·ÖŹż £ØŃ”Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°²»±ä”±£©”£
[ŹµŃé·ÖĪö]·½°øI²ā³öµÄŃõ»ÆĢśµÄÖŹĮæ·ÖŹżĆ÷ĻŌ“óÓŚ·½°ø¢ņ²ā³öµÄ½į¹ū£¬æÉÄܵÄŌŅņŹĒ £ØŠ“³öŅ»ÖÖ¼“æÉ£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
£Ø7·Ö£©ŌŚŅ»ÉÕ±ÖŠŹ¢ÓŠ60.2gBaCO3ŗĶBaCl2µÄ·Ūĩד»ģŗĻĪļ£¬ĻņĘäÖŠ¼ÓČė188.8gĖ®Ź¹»ģŗĻĪļÖŠæÉČÜĪļĶźČ«Čܽā£¬Č»ŗóĻņĘäÖŠÖšµĪ¼ÓČėČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄĻ”ŃĪĖįÖĮ146gŹ±Ē”ŗĆ·“Ó¦ĶźČ«£¬Ēėøł¾ŻĢāŅā»Ų“šĪŹĢā£ŗ
£Ø1£©ŌŚµĪ¼ÓŃĪĖį¹ż³ĢÖŠ¹Ū²ģµ½µÄĆ÷ĻŌŹµŃéĻÖĻóŹĒ ”£
£Ø2£©µ±ŃĪĖįµĪ¼ÓÖĮ140gŹ±£¬ÉÕ±ÖŠČÜŅŗĄļŗ¬ÓŠČÜÖŹµÄŹĒ ”££ØŠ“»ÆѧŹ½£©
£Ø3£©¼ĘĖćĒ”ŗĆ·“Ó¦ĶźČ«Ź±ÉÕ±ÖŠĖłµĆ²»±„ŗĶČÜŅŗµÄÖŹĮ攣£Ø½į¹ū¾«Č·µ½0.1g£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
£Ø7·Ö£©ÖŠŗĶ·“Ó¦ŹĒ֊ѧ»Æѧ֊ÖŲŅŖµÄѧĻ°ÄŚČŻ£¬ĘäŌŚČÕ³£Éś»īŗĶ¹¤Å©ŅµÉś²śÖŠÓŠ¹ć·ŗµÄÓ¦ÓĆ”£
£Ø1£©ĻĀĶ¼±ķŹ¾ŃĪĖįŗĶĒāŃõ»ÆÄĘČÜŅŗ·¢Éś·“Ó¦¹ż³ĢÖŠČÜŅŗµÄpHµÄ±ä»ÆĒśĻß”£Ēė“ÓĒśĻßĶ¼ÖŠ»ńČ”ŠÅĻ¢£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ŁĶ¼1Ķ¼Ļó±ķŹ¾ŃĪĖįŗĶĒāŃõ»ÆÄĘČÜŅŗ·¢Éś·“Ó¦¹ż³ĢÖŠČÜŅŗµÄpH±ä»Æ”£½ųŠŠøĆ·“Ó¦µÄŹµŃé²Ł×÷ŹĒ°“ÕÕĶ¼2ÖŠµÄ £ØĢī¼×»ņŅŅ£©Ķ¼ĖłŹ¾½ųŠŠµÄ”£
¢ŚĒśĻßÉĻMµć±ķŹ¾ ”£
¢ŪĻņÉÕ±ÖŠĒćµ¹20gÖŹĮæ·ÖŹżĪŖ4.00£„µÄĒāŃõ»ÆÄĘČÜŅŗ£¬µĪČė3µĪ·ÓĢŖŹŌŅŗ£¬Õńµ“£¬ŌŁÖšµĪµĪČėÖŹĮæ·ÖŹżĪŖ3.65£„µÄĻ”ŃĪĖį£¬±ßµĪ±ßÕńµ“£¬Ö±ÖĮČÜŅŗøÕŗƱäĪŖ É«ĪŖÖ¹£¬¹²ÓĆČ„Ļ”ŃĪĖį20g£¬Ōņ·“Ó¦ŗóČÜŅŗÖŠČÜÖŹÖŹĮæ·ÖŹżĪŖ (½į¹ū¾«Č·µ½0.1£„)”£
£Ø2£©ĪŖÖ¤Ć÷ÖŠŗĶ·“Ó¦ŹĒ·ÅČČ·“Ó¦£¬Ä³Š”×é½ųŠŠĮĖČēĶ¼Ėł
Ź¾µÄŹµŃé²Ł×÷£ŗ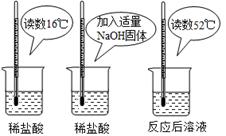
øł¾ŻÉĻĶ¼ŹµŃ飬¼×Ķ¬Ń§ČĻĪŖ£ŗNaOHÓėĻ”ŃĪĖį·¢ÉśµÄÖŠŗĶ·“Ó¦ŹĒ·ÅČČ·“Ó¦”£ŅŅĶ¬Ń§ČĻĪŖ£ŗ¼×Ķ¬Ń§µĆ³ö
Õāøö½įĀŪµÄŅĄ¾Ż²»æĘѧ£¬ĄķÓÉŹĒ ”£
£Ø3£©ĪŖĢ½¾æÓ°ĻģÖŠŗĶ·“Ó¦·Å³öČČĮæ¶ąÉŁµÄŅņĖŲ£¬ĖūĆĒÓÖ½ųŠŠĮĖČēĻĀŹµŃé£ŗŌŚ±ąŗÅĪŖA”¢B”¢C”¢
D”¢EµÄĪåÖ»ÉÕ±ÖŠø÷×°Čė36.5g ČÜÖŹÖŹĮæ·ÖŹżĪŖ5%”¢10%”¢15%”¢20%”¢25%µÄŃĪĖį£¬
ŌŁĻņÉĻŹöĪåÖ»ÉÕ±ÖŠ·Ö±š¼ÓČė40g20% µÄĒāŃõ»ÆÄĘČÜŅŗ£¬×īŗó²āĮæĘäĪĀ¶Č£¬Źż¾Ż¼ĒĀ¼ČēĻĀ£ŗ
| ÉÕ±±ąŗÅ | A | B | C | D | E |
| ŃĪĖįµÄČÜÖŹÖŹĮæ·ÖŹż | 5% | 10% | 15% | 20% | 25% |
| ·“Ó¦ŗóČÜŅŗĪĀ¶Č£Ø”ę£© | 24”ę | 34”ę | 46”ę | 54”ę | 54”ę |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
£Ø7 ·Ö£©ŃĪĖ®Ń”ÖÖŹĒĪŅ¹ś¹Å“śĄĶ¶ÆČĖĆń·¢Ć÷µÄŅ»ÖÖĒÉĆīµÄĢōŃ”ÖÖ×ӵķ½·Ø”£Å©ŅµÉś²śÉĻ³£ÓĆÖŹĮæ·ÖŹż 15%”Ŗ20%µÄĀČ»ÆÄĘČÜŅŗĄ“Ń”ÖÖ”£ĪŖĮĖ²ā¶ØijĀČ»ÆÄĘČÜŅŗŹĒ·ń·ūŗĻŅŖĒó£¬Č”øĆČÜŅŗ70g£¬¼ÓČėŅ»¶ØČÜÖŹÖŹĮæ·ÖŹżµÄ AgNO3ČÜŅŗ 100g£¬Ē”ŗĆĶźČ«·“Ó¦£¬¹żĀĖ·ÖĄė³ö³ĮµķµÄÖŹĮæĪŖ28£®7g”£
£Ø1£©ŌŚÅäÖĘĀČ»ÆÄĘČÜŅŗµÄ²Ł×÷¹ż³ĢÖŠ£¬ČܽāŹ±ŠčŅŖÓƵ½²£Į§°ō£¬Ęä×÷ÓĆŹĒ ”£
£Ø2£©·“Ó¦ŗóĖłµĆČÜŅŗµÄÖŹĮæĪŖ g”£
£Ø3£©Ķعż¼ĘĖćČ·¶ØøĆĀČ»ÆÄĘČÜŅŗŹĒ·ń·ūŗĻŃ”ÖÖŅŖĒó£æ£Ø¼ĘĖć½į¹ū¾«Č·µ½0£®1%£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
£Ø3·Ö£©Įņ“śĮņĖįÄĘ£ØNa2S2O3£©ŹĒŅ»ÖÖÓĆĶ¾¹ć·ŗµÄĪļÖŹ”£Ä³Įņ“śĮņĖįÄĘѳʷ֊ŗ¬ÓŠÉŁĮæµÄĮņĖįÄĘ”£ĻÖČ”16 gøĆѳʷ·ÅČėÉÕ±ÖŠ£¬¼ÓČė113.6 gŅ»¶ØČÜÖŹÖŹĮæ·ÖŹżµÄĻ”ĮņĖįĒ”ŗĆĶźČ«·“Ó¦£¬µĆµ½120 gĮņĖįÄĘ²»±„ŗĶČÜŅŗ”£
·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗNa2S2O3 + H2SO4=== Na2SO4 + H2O + S”ż+ SO2”ü
Ēė¼ĘĖć£ŗ
£Ø1£©ŃłĘ·ÖŠĮņ“śĮņĖįÄĘ£ØNa2S2O3£©ÓėĮņĖįÄʵÄÖŹĮæ±Č”£
£Ø2£©ĖłµĆČÜŅŗÖŠČÜÖŹÖŹĮæ·ÖŹż”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
£Ø3·Ö£©Ä³»Æ¹¤³§Éś²śµÄŃõ»ÆŃĒĶ£ØCu2O£©²śĘ·ÖŠ³£ŗ¬ÓŠ10%µÄ½šŹōĶ”£ĻÖ×¼±øÓĆ40 tøĆ²śĘ·Éś²ś10%µÄĮņĖįĶČÜŅŗ”£ŅŃÖŖ£ŗCu2O+H2SO4===CuSO4+Cu+H2O
Ēó£ŗĖłŠčČÜÖŹÖŹĮæ·ÖŹżĪŖ20%µÄĻ”ĮņĖįÖŹĮæŗĶ¼ÓĖ®µÄÖŹĮ攣
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
Ź¹ĢśŗĶĶµÄ»ģŗĻĪļ 20 gÓė×ćĮæµÄĻ”ĮņĖį·“Ó¦£¬æÉÖʵĆĒāĘų0.4 g£¬ĒóŌ»ģŗĻĪļÖŠĢśŗĶĶø÷ĪŖ¶ąÉŁæĖ£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
øÖĢś³§¹ŗ½ųŗ¬Ńõ»ÆĢś85%µÄ³ąĢśæó1000t£¬×¼±øÓĆÕāŠ©ĢśæóŹÆĮ¶Č”ŗ¬ŌÓÖŹ3%µÄÉśĢś£¬ĒėĄūÓĆ»Æѧ·½³ĢŹ½¼ĘĖćÉśĢśµÄÖŹĮæ £Ø¼ĘĖć½į¹ū±£ĮōÕūŹż£©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com