
��9�֣�ˮ����ͨ�����ȵĽ�̿��õ��Ļ����������Щ�ɷ֣�ij��ѧ��ȤС������ʦ��ָ���£��Դ˽�����ʵ��̽����
��������롿1���û������ֻ����һ����̼������
2���û�����庬��һ����̼��������̼��������ˮ����
3���û������ֻ���ж�����̼��������ˮ����
4���û������ֻ����һ����̼��������̼������
���������ϡ�a����ˮ����ͭ��ˮ�ɰ�ɫ��Ϊ��ɫ��
b����ʯ���ǹ����������ƺ������ƵĻ���
c��Ũ�������ǿ�ҵ���ˮ�ԣ�������ijЩ����ĸ����
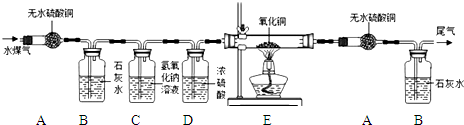
��ʵ����̡�ͬѧ������ʦ��ָ�������������ͼ��ʾװ�ã���������ʵ��(���ּг���������ȥ)��
��1��װ��A����ˮ����ͭ������װ��B�г���ʯ��ˮ����ǣ��ɴ˵ó��Ľ���Ϊ����������� ��B�б仯�Ļ�ѧ����ʽΪ ��
��2��װ��C�е�ҩƷΪ ��
��3��E������ͭ��졢F����ˮ����ͭ������G�г���ʯ��ˮ����ǣ�˵����������л����ڵ������� �� E�еı仯˵������ͭ���� �ԡ�
��ʵ����ۡ����� ��ȷ��
��ʵ�鷴˼��
�������ۣ�ͬѧ�ǽ���ͼ��װ��C~H�����˼�ֻ ����ͼ��ʾװ�ò���ѡ��Ҫ�Լ��������̽�������У�������м�ʯ�ҵ�����Ϊ �� �ձ����ܹ۲쵽�������� �����һ����ʵ������������� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


| ʵ����� | ʵ������ | ʵ����� | |
| �� | �ڼ��촦��ȼ���壬 �ڻ����Ϸ���һ�� ������ྻ���ձ� |
�ձ��ڱ� ������ɫҺ�� |
�������������� �� |
| �� | �ڻ����Ϸ���һ��Ϳ ��������ʯ��ˮ���ձ� |
�ձ��ڳ��� ��ʯ��ˮ����� |
��������к���һ����̼ �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||

| ʵ����� | ���� | ���� |
ȡ�����Ĺ���̼�������Թ��У�����������ˮ���������ǵ�ľ�������Թܣ� ȡ�����Ĺ���̼�������Թ��У�����������ˮ���������ǵ�ľ�������Թܣ� |
�д������ݲ����������ǵ�ľ����ȼ �д������ݲ����������ǵ�ľ����ȼ |
����̼����������ˮ��Ѹ�ٷų����� ����̼����������ˮ��Ѹ�ٷų����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com