
Š”Ć÷Ķ¬Ń§ŌŚÕūĄķ³ų·æŹ±²»Š”ŠÄ½«Ņ»Ęæ“דņ·£¬“×Č÷ŌŚ»šĀÆÅŌµÄŅ»¶Ń²ŻÄ¾»ŅÉĻ£¬·¢ĻÖÓŠ“óĮæµÄĘųÅŻÉś³É”£¼¤·¢ĮĖŠĖȤ,ÓŚŹĒĖū¾ö¶ØÓėѧĻ°Š”×éµÄĶ¬Ń§£¬¶Ō²ŻÄ¾»ŅµÄ³É·Ö½ųŠŠĢ½¾æ”£
[Ģ½¾æ»ī¶ÆŅ»] øł¾ŻĖłŃ§Ėį¼īŃĪÖŖŹ¶£¬Ķ¬Ń§ĆĒ²ĀĻė²ŻÄ¾»ŅÖŠŗ¬ÓŠÄÜÓėĖį·“Ó¦²śÉśĘųĢåµÄŃĪĄąĪļÖŹ£¬ÓŚŹĒ£¬Éč¼Ę²¢½ųŠŠČēĻĀĶ¼ĖłŹ¾µÄŹµŃ锣¹Ū²ģµ½×¶ŠĪĘæAÄŚÓŠ“óĮæµÄĘųÅŻĆ°³ö£¬ŹŌ¹ÜBÖŠ³ĪĒåµÄŹÆ»ŅĖ®±ä»ė×Ē”£
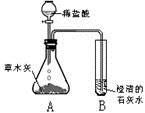
øł¾ŻÉĻŹöŹµŃéĻÖĻóĶʶĻ£ŗ²śÉśµÄĘųĢåÖŠŗ¬ÓŠ £¬
ŹŌ¹ÜBÖŠÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
[Ģ½¾æ»ī¶Æ¶ž]׏ĮĻÕŖŅŖ¢ń£ŗ²ŻÄ¾»ŅÖŠµÄÖ÷ŅŖ³É·ÖŹĒĢ¼Ėį¼Ų£¬Ģ¼Ėį
¼ŲµÄĖ®ČÜŅŗŗĶĢ¼ĖįÄĘČÜŅŗĖį¼īŠŌĻąĖĘ”£
£Ø1£©ĻņĢ¼Ėį¼ŲµÄĖ®ČÜŅŗÖŠµĪČė·ÓĢŖŹŌŅŗ£¬ČÜŅŗĻŌ É«”£
£Ø2£©ĒėÄ抓³öÉĻŹö”°Ģ½¾æ»ī¶ÆŅ»”±ÖŠ£¬×¶ŠĪĘæAÄŚ·¢ÉśµÄŅ»øö·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø3£©Ä³Ķ¬Ń§Óū²ā¶ØÕāŠ©²ŻÄ¾»ŅÖŠĢ¼Ėį¼ŲµÄŗ¬Į棬³ĘĢģĘ½³ĘČ”69gŹŌŃłÓŚÉÕ±ÖŠ£¬¼ÓČė100gĻ”ŃĪĖįĒ”ŗĆĶźČ«·“Ó¦£¬³ä·Ö·“Ó¦ŗó£¬³ĘµĆ»ģŗĻĪļµÄ×ÜÖŹĮæĪŖ164.6 g£ØŗöĀŌ¶žŃõ»ÆĢ¼µÄČܽā¶ŌÖŹĮæµÄÓ°Ļģ£©”£Ēė¼ĘĖć£ŗ
¢Ł·“Ó¦¹ż³ĢÖŠ²śÉśµÄ¶žŃõ»ÆĢ¼ÖŹĮæĪŖ g”£¢ŚøĆ²ŻÄ¾»ŅŹŌŃłÖŠĢ¼Ėį¼ŲµÄÖŹĮæ·ÖŹż”£
¢ŪČōÅäÖĘŹµŃéÖŠĖłÓƵÄĻ”ŃĪĖį100g£¬ŠčŅŖČÜÖŹÖŹĮæ·ÖŹżĪŖ14.6%µÄŃĪĖį¶ąÉŁæĖ£æ
[Ģ½¾æ»ī¶ÆŅ»]
¶žŃõ»ÆĢ¼£Ø»ņCO2£© £Ø1·Ö£©
Ca£ØOH£©2+CO2====CaCO3”ż+H2O £Ø2·Ö£©
[Ģ½¾æ»ī¶Æ¶ž]
£Ø1£©ŗģ £Ø1·Ö£©
£Ø2£©K2CO3£«2HCl£½2KCl£«H2O£«CO2”ü £Ø2·Ö£©
£Ø3£©¢Ł4.4 g ” ””” ”” ”£Ø1·Ö£©
¢Ś£Ø4·Ö£©½ā£ŗÉčŹŌŃłÖŠK2CO3µÄÖŹĮæĪŖx£¬ĻūŗÄHClµÄÖŹĮæĪŖy
K2CO3+2HCl=2KCl+H2O+CO2ӟ
138 73 44
x y 4.4 g ””””””””” ””£Ø1·Ö£©
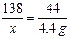 ½āµĆ£ŗx="13.8" g ”””” ””£Ø1·Ö£©
½āµĆ£ŗx="13.8" g ”””” ””£Ø1·Ö£©
 ½āµĆ£ŗx="7.3" g ””””””£Ø1·Ö£©
½āµĆ£ŗx="7.3" g ””””””£Ø1·Ö£©
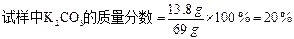 ”””””” ”£Ø1·Ö£©
”””””” ”£Ø1·Ö£©
¢ŪÉčŠčŅŖČÜÖŹÖŹĮæ·ÖŹżĪŖ14.6%µÄŃĪĖįÖŹĮæĪŖZ
Z”Į14.6%==7.3g ½āµĆ£ŗ Z==50g £Ø1·Ö£©


 ѧŅµ²āĘĄŅ»æĪŅ»²āĻµĮŠ“š°ø
ѧŅµ²āĘĄŅ»æĪŅ»²āĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
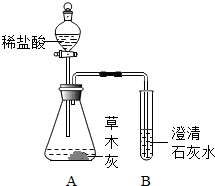 £Ø2012?ŃĪ³ĒÄ£Äā£©Š”Ć÷Ķ¬Ń§ŌŚÕūĄķ³ų·æŹ±²»Š”ŠÄ½«Ņ»Ęæ“דņ·£¬“×Č÷ŌŚ»šĀÆÅŌµÄŅ»¶Ń²ŻÄ¾»ŅÉĻ£¬·¢ĻÖÓŠ“óĮæµÄĘųÅŻÉś³É£®¼¤·¢ĮĖŠĖȤ£¬ÓŚŹĒĖū¾ö¶ØÓėѧĻ°Š”×éµÄĶ¬Ń§£¬¶Ō²ŻÄ¾»ŅµÄ³É·Ö½ųŠŠĢ½¾æ£®
£Ø2012?ŃĪ³ĒÄ£Äā£©Š”Ć÷Ķ¬Ń§ŌŚÕūĄķ³ų·æŹ±²»Š”ŠÄ½«Ņ»Ęæ“דņ·£¬“×Č÷ŌŚ»šĀÆÅŌµÄŅ»¶Ń²ŻÄ¾»ŅÉĻ£¬·¢ĻÖÓŠ“óĮæµÄĘųÅŻÉś³É£®¼¤·¢ĮĖŠĖȤ£¬ÓŚŹĒĖū¾ö¶ØÓėѧĻ°Š”×éµÄĶ¬Ń§£¬¶Ō²ŻÄ¾»ŅµÄ³É·Ö½ųŠŠĢ½¾æ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 Š”Ć÷Ķ¬Ń§ŌŚÕūĄķ³ų·æŹ±²»Š”ŠÄ½«Ņ»Ęæ“דņ·£¬“×Č÷ŌŚ»šĀÆÅŌµÄŅ»¶Ń²ŻÄ¾»ŅÉĻ£¬·¢ĻÖÓŠ“óĮæµÄĘųÅŻÉś³É£®¼¤·¢ĮĖŠĖȤ£¬ÓŚŹĒĖū¾ö¶ØÓėѧĻ°Š”×éµÄĶ¬Ń§£¬¶Ō²ŻÄ¾»ŅµÄ³É·Ö½ųŠŠĢ½¾æ£®
Š”Ć÷Ķ¬Ń§ŌŚÕūĄķ³ų·æŹ±²»Š”ŠÄ½«Ņ»Ęæ“דņ·£¬“×Č÷ŌŚ»šĀÆÅŌµÄŅ»¶Ń²ŻÄ¾»ŅÉĻ£¬·¢ĻÖÓŠ“óĮæµÄĘųÅŻÉś³É£®¼¤·¢ĮĖŠĖȤ£¬ÓŚŹĒĖū¾ö¶ØÓėѧĻ°Š”×éµÄĶ¬Ń§£¬¶Ō²ŻÄ¾»ŅµÄ³É·Ö½ųŠŠĢ½¾æ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ½ĖÕÄ£ÄāĢā ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com