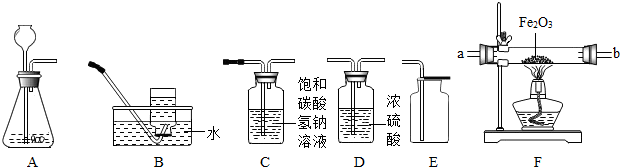
��1��ʵ���ҳ�����ͼA��Bװ��֧ȡ���壬�ش��������⣺
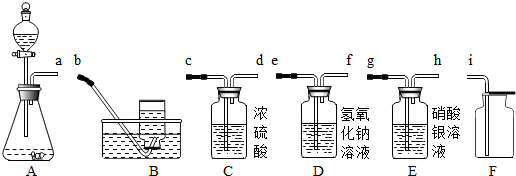
�ٲ�������a��b��c�����Ʒֱ���
�Թ�
�Թ�
��
�ƾ���
�ƾ���
��
����ƿ
����ƿ
��
��С����ȡ������̼���壬Ӧѡ��ͼ�е�
A
A
װ�ã�����ĸ����ʹ�õ�ҩƷ��
����ʯ����ʯ��ʯ��
����ʯ����ʯ��ʯ��
��
ϡ����
ϡ����
��д���÷�Ӧ�Ļ�ѧ����ʽ
CaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
��
��С��Ҫ��Aװ����ȡ��������壬��ƿ��1��Ӧʢ��
C
C
������ţ�
A����������Һ BŨ���� CŨ����
��д����Bװ����ȡ�����һ����ѧ����ʽ
��
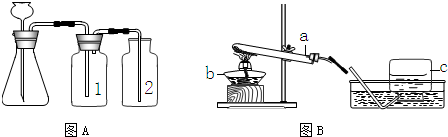
��Aװ�������Եļ��飺�ֱ��ˮ��û����ƿ2�е��ܵ�ĩ�˺���ƿ�г���©����ĩ�ˣ�Ȼ������ë����סϴ��ƿ1��������ƿ2��
���ܿ�û������ð��
���ܿ�û������ð��
����ƿ��
Һ�治�½�������©������ˮ������������©������Һ������ƿ��Һ�治�γ�Һ��
Һ�治�½�������©������ˮ������������©������Һ������ƿ��Һ�治�γ�Һ��
�����װ��©����
��2��ijѧУʵ���ҵķ�Һ�����ռ���ѧ����ʵ������ȡ������̼������ķ�Һ��С��ͬѧ��̽����Һ�����ʵijɷ֣�����һͬ����̽�����ش��������⣺
��Һ�е�������ʲô���ʣ�
С����Ϊ��Һ�е�����ֻ��CaCl
2������Ϊ�����ܺ��е�������
HCl
HCl
�����ѧʽ����
CaCl
2��Һ�����ԣ�
ʵ������ƣ���1��С��ȡ����������Һ�������Ȼ�����Һ�ֱ���뵽��֧�Թ��У����ֱ������ɫ��̪��Һ���Ա����飬�����֧�Թ�����Һ����ɫ������С����Ϊ�Լ��IJ�������ȷ�ģ�
��2������ΪС���ʵ��
����
����
����ܡ����ܡ���֤�����IJ�������ȷ�ģ�������
��̪������Ҳ����ɫ����ȷ���Ƿ���HCl
��̪������Ҳ����ɫ����ȷ���Ƿ���HCl
��
��3�����Ҫ֤����IJ�������ȷ�ģ���ѡ����Լ���
��ɫʯ����Һ
��ɫʯ����Һ
����������Լ�ʱ�۲쵽��������
��Һ���
��Һ���
��
��չӦ�ã���1����ʵ��֤����IJ�������ȷ�ģ���Ҫ������Һֻ�õ�CaCl
2��Һ��Ӧ���Һ�м��������
CaCO3
CaCO3
����Ӧ��ȫ����ˣ�
��2��ͨ������̽������Ϊʵ���ҵķ�Һδ������ֱ�ӵ�����ˮ����������ɵ�Σ����
���ܸ�ʴ������ˮ�ܵ�������Ⱦˮ�ʣ�
���ܸ�ʴ������ˮ�ܵ�������Ⱦˮ�ʣ�
����дһ����

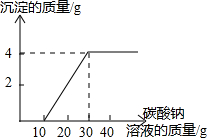 ʵ������ȡ������̼�Ի��յ�������Ȼ��ƻ����Һ���������������ʣ�����������ʵ�飺ȡ100g����Һ���ձ��У�����30g������������Ϊ21.2%��̼������Һ����ַ�Ӧ����ˣ�����̼������Һ��������������������Ĺ�ϵ��ͼ��ʾ����
ʵ������ȡ������̼�Ի��յ�������Ȼ��ƻ����Һ���������������ʣ�����������ʵ�飺ȡ100g����Һ���ձ��У�����30g������������Ϊ21.2%��̼������Һ����ַ�Ӧ����ˣ�����̼������Һ��������������������Ĺ�ϵ��ͼ��ʾ����