
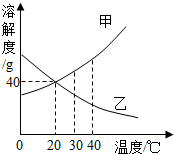
 ��ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ����ͼ�ش��������⣮
��ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ����ͼ�ش��������⣮| �������� |
| ��������+�ܼ����� |


 ��������ϵ�д�
��������ϵ�д� ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ʳ���ݵķ�������ȥˮ���е�ˮ�� |
| B����п����Ũ���������Լ���ȡ���﴿�������� |
| C����ϡ���������Һ��������̼�������Ƿ��ж����������� |
| D��������Һ�м�������Ũ���Ტ���Լ��鵰���ʵĴ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������A | B������B |
| C������C | D������D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
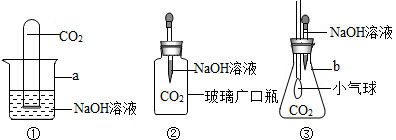
| �������������������д��ʵ��������û�У�������Ľ������� | |
| ʵ��� | |
| ʵ��� | |
| ʵ��� |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ����1����NaOH��Һ��ͨ��CO2���� ����2����������Һ�е���CaCl2��Һ | ����1��û���������� ����2������ ��Ӧ����ʽΪ | CO2��NaOHȷʵ�����˻�ѧ��Ӧ |
| ʵ�鲽�� | ʵ������ | ���� |
| CO2��NaOHȷʵ�����˻�ѧ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ�� A��B��C ���ֹ������ʵ��ܽ������ͼ������ͼʾ�ش��������⣺
��ͼ�� A��B��C ���ֹ������ʵ��ܽ������ͼ������ͼʾ�ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
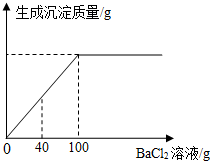 С��ͬѧΪ�˲ⶨij������Na2CO3��NaOH����̼���Ƶ���������������������ʵ�飺�ֳ�ȡ�˸���Ʒ20g�����Һ��Ȼ�������Һ����μ���������������Ϊ20.8%��BaCl2��Һ�����ɳ��������������BaCl2��Һ��������ϵ��ͼ��ʾ��
С��ͬѧΪ�˲ⶨij������Na2CO3��NaOH����̼���Ƶ���������������������ʵ�飺�ֳ�ȡ�˸���Ʒ20g�����Һ��Ȼ�������Һ����μ���������������Ϊ20.8%��BaCl2��Һ�����ɳ��������������BaCl2��Һ��������ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
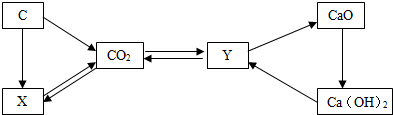
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com