
���� ��1��ú��ʯ�ͺ���Ȼ�����ڻ�ʯȼ�ϣ��Dz���������Դ������ȼ������ˮ�Ͷ�����̼��
��2�����������ǵ��������һ�����ʣ�
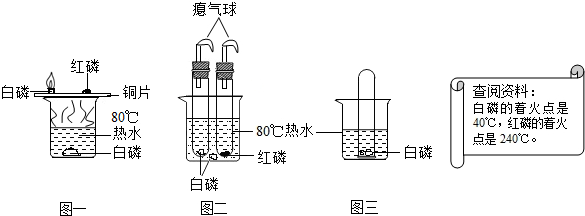
��3���ٶԱ�ͼһ��ͼ����ͼ���������ܱյ��Թ��н���ʵ�飬���Է�ֹ���ɵ�������������ɿ�����Ⱦ��
�ڰ����¶ȴﵽ���Ż���û�ܺ������Ӵ���ȼ����Ҫ����֧�֣��Թ���Ӧ�ṩȼ����Ҫ��������
��� �⣺��1��Ŀǰ��������������Դ��ú��ʯ�ͺ���Ȼ���Ȼ�ʯȼ�ϣ���Ȼ���е���Ҫ�ɷ��Ǽ��飬����ȼ�յĻ�ѧ����ʽΪ��CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O��
��2��úȼ�ջ����������CO2��SO2��CO�����壬���лᵼ�������������SO2�����δ�ʩΪ��ҵ�����辻���������������ŷţ���ֹ��ɴ�����Ⱦ���˷�����Դ��
��3����ͼ���������ܱյ��Թ��У����Է�ֹȼ�պ����ɵ�������������Ⱦ��������ͼһ��ȼ�ղ���ֱ�����������
���Թ���ӦΪ��������������������ȼ������Ҫ��������
�ʴ�Ϊ����������������
�𰸣�
��1��ʯ�ͣ�CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O��
��2��SO2����ҵ�����辻���������������ŷţ�
��3���ټ�����Ⱦ����������
��O2
���� ��ʯȼ�ϵ�ȼ���ܹ����������Ķ��������������Щ�������γ��������Ҫ���ʣ���˼��ٻ�ʯȼ�ϵ�ʹ�ã��ۺ����û�ʯȼ�������ڼ�������IJ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ǧ����⣬����ѩƮ�����ԡ�����•ѩ���� | |
| B�� | ���ϵ���˿����������ɻ���ʼ�ɣ����ԡ����⡷�� | |
| C�� | ����ֱ����ǧ�ߣ�������������죨���ԣ�����®ɽ�ٲ����� | |
| D�� | ����Ϊ�������������ԡ���¥�Ρ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
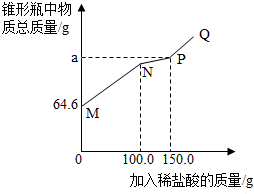 ȡ14.6g���ʵ��������ƹ�����Ʒ������ˮ������ƿ�У�����50.0gˮ������ܽ⣬������ƿ�еμӵ�������������Ϊ7.3%��ϡ���ᣮʵ���ü���ϡ�������������ƿ�����ʵ���������ϵ��ͼ��ʾ����̼������ϡ���ᷴӦ���������У�Na2CO3+HCl�TNaHCO3+NaCl��NaHCO3+HCl�TNaCl+H2O+CO2��������˵����ȷ���ǣ�������
ȡ14.6g���ʵ��������ƹ�����Ʒ������ˮ������ƿ�У�����50.0gˮ������ܽ⣬������ƿ�еμӵ�������������Ϊ7.3%��ϡ���ᣮʵ���ü���ϡ�������������ƿ�����ʵ���������ϵ��ͼ��ʾ����̼������ϡ���ᷴӦ���������У�Na2CO3+HCl�TNaHCO3+NaCl��NaHCO3+HCl�TNaCl+H2O+CO2��������˵����ȷ���ǣ�������| A�� | N�����Һ��ʾ�������Ʊ�ǡ����ȫ��Ӧ | |
| B�� | PQ�Σ�����P�㣩��Һ��pH��7 | |
| C�� | NP�α�ʾ��������Ĺ��̣�a��ֵΪ201.2 | |
| D�� | �ù�����Ʒ���������Ƶ�����Ϊ8.0g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| ��������������Һ�������ˣ� | 40 | 40 | 40 | 30 |
| ���ɳ��������������ˣ� | 0 | 4.9 | 9.8 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
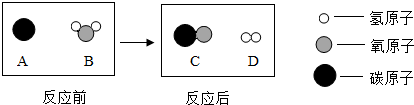
| ��ɫ����ζ | �۵㣨�棩 | �е㣨�棩 | ����ʱ��1Lˮ�����ܽ��������� | ������ܶȡ���g/L�� | |
| NH3 | ��ɫ���̼��� | -77.7 | -33 | 700L | 0.6942 |
| H2 | ��ɫ����ζ | -259.2 | -253 | 0.017L | 0.0899 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com