
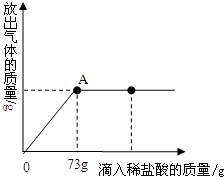
 ��ʢ��22.3g Na2CO3��NaCl��ɵĻ�Ϲ�����ձ��У�����ܽ��143.1g��ˮ��Ȼ���������μ�������������Ϊ10%��ϡ���ᣨ��֪�Ȼ�����ϡ�����Ӧ�����ų��������������������ϡ�����������ϵ������ͼ��ʾ������㣺
��ʢ��22.3g Na2CO3��NaCl��ɵĻ�Ϲ�����ձ��У�����ܽ��143.1g��ˮ��Ȼ���������μ�������������Ϊ10%��ϡ���ᣨ��֪�Ȼ�����ϡ�����Ӧ�����ų��������������������ϡ�����������ϵ������ͼ��ʾ������㣺| 106 |
| x |
| 73 |
| 7.3g |
| 117 |
| y |
| 44 |
| z |
| 23.4g |
| 234g |


 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
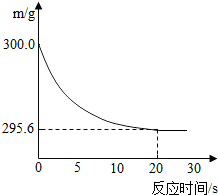 ��ʢ��22.3g Na2CO3��NaCl����������ձ��м���216.1gϡ����ǡ�÷�Ӧ����Ӧ�����þ�����������ձ���ͬҩƷ��������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ���ձ���ͬҩƷ����ʼ����Ϊ300g�����з���������������ǣ�������
��ʢ��22.3g Na2CO3��NaCl����������ձ��м���216.1gϡ����ǡ�÷�Ӧ����Ӧ�����þ�����������ձ���ͬҩƷ��������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ���ձ���ͬҩƷ����ʼ����Ϊ300g�����з���������������ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ģ���� ���ͣ�������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ���е�ͽ�����꼶���ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�꽭��ʡ���Ƹ����п���ѧģ���Ծ��������������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com