
��2012?����������ͬѧ�Բ�ͬ�Ļ�ѧ˼ά��ʽ���߹��ɸ����л�ѧ��Ӧ�����ͽ��з��࣬����Ϊ���������һ���ǣ���������
��CaO+H2O�TCa��OH��2��
��Mg��OH��2 MgO+H2O��
MgO+H2O��
��Ba��OH��2+2HCl�TBaCl2+2H2O��
��Zn+H2SO4�TZnSO4+H2����
��2Mg+O2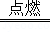 2MgO��
2MgO��
| �� | A�� | ���ڻ��Ϸ�Ӧ���Ǣ٢� | B�� | ���ڸ��ֽⷴӦ���Ǣۢ� |
| �� | C�� | �����������ų��ķ�Ӧ���Ǣڢ� | D�� | ������Ԫ�ػ��ϼ۱仯���Ǣܢ� |
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д� ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����б�ҵ��ѧ���ԣ��Ĵ�����������ѧ�������棩 ���ͣ�ѡ����
��2012•����������ͬѧ�Բ�ͬ�Ļ�ѧ˼ά��ʽ���߹��ɸ����л�ѧ��Ӧ�����ͽ��з��࣬����Ϊ���������һ���ǣ���������
��CaO+H2O�TCa��OH��2��
��Mg��OH��2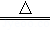 MgO+H2O��
MgO+H2O��
��Ba��OH��2+2HCl�TBaCl2+2H2O��
��Zn+H2SO4�TZnSO4+H2����
��2Mg+O2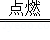 2MgO��
2MgO��
|
�� |
A�� |
���ڻ��Ϸ�Ӧ���Ǣ٢� |
B�� |
���ڸ��ֽⷴӦ���Ǣۢ� |
|
�� |
C�� |
�����������ų��ķ�Ӧ���Ǣڢ� |
D�� |
������Ԫ�ػ��ϼ۱仯���Ǣܢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com