
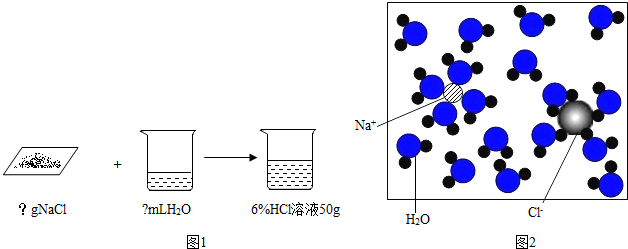
·ÖĪö £Ø1£©øł¾ŻČÜÖŹÖŹĮæ=ČÜŅŗÖŹĮæ”ĮČÜÖŹµÄÖŹĮæ·ÖŹż£¬ČܼĮÖŹĮæ=ČÜŅŗÖŹĮæ-ČÜÖŹÖŹĮ棬ĄūÓĆv=$\frac{m}{¦Ń}$£¬¼ĘĖćĢå»ż¼“æÉ£»
£Ø2£©øł¾ŻĢģĘ½µÄŹ¹ÓĆ×¢ŅāŹĀĻī½ā“š£»
£Ø3£©ĮæČ”Ė®Ź±£¬Ó¦Ń”ÓĆĮæ³Ģ×ī½Ó½üµÄ£»
£Ø4£©ČܽāŹ±²£Į§°ōµÄ×÷ÓĆ½ā“š£»
£Ø5£©øł¾ŻČÜŅŗŹŌ¼ĮĘæ±źĒ©Ó¦³öŹ¾Ź²Ć“ČÜŅŗŗĶÅØ¶Č“óŠ”½ā“š£»
£Ø6£©øł¾ŻÅäÖĘČÜŅŗŹ±Ōģ³ÉĀČ»ÆÄʵÄÖŹĮæ·ÖŹżĘ«Š”µÄŌŅņ½ā“š£»
½ā“š ½ā£ŗ£Ø1£©ÅäÖĘ50gČÜÖŹÖŹĮæ·ÖŹżĪŖ6%µÄĀČ»ÆÄĘČÜŅŗŠčŅŖĀČ»ÆÄĘ£ŗ50g”Į6%=3g£»ŠčĖ®£ŗ$\frac{50g-3g}{1g/mL}$=47ml£»
£Ø2£©ÓĆĶŠÅĢĢģĘ½³ĘĮæĖłŠčµÄĀČ»ÆÄĘŹ±£¬·¢ĻÖĢģĘ½µÄÖøÕėĘ«Ļņ×óÅĢ£¬ĖµĆ÷ĀČ»ÆÄĘ³¬ĮæÓ¦¼õÉŁŹŹĮæĀČ»ÆÄĘ¹ĢĢ壻ČōŌŚ³ĘČ”ĀČ»ÆÄĘµÄ²Ł×÷ÖŠ£¬ÖøÕėĀŌĻņÓŅĘ«×Ŗ£¬ĖµĆ÷Ņ©Ę·ÉŁ£¬ŅŖŹ¹ÖøÕė¾ÓÖŠµÄĒ”µ±²Ł×÷ŹĒ£ŗ¼ĢŠųĢķ¼ÓĀČ»ÆÄĘ£¬Ö±µ½ĢģĘ½Ę½ŗā£»
£Ø3£©ĪŖ¼õÉŁĪó²ī£¬ĮæČ”Ė®Ź±£¬Ó¦Ń”ÓĆĮæ³Ģ×ī½Ó½üµÄ£¬¹ŹÓ¦Ń”50mLµÄĮæĶ²£»
£Ø4£©ČܽāŹ±ŅŖÓĆ²£Į§°ō½Į°č£¬×÷ÓĆŹĒ¼ÓĖŁĀČ»ÆÄĘČܽā£»ÓÉĶ¼Ź¾æÉÖŖ£ŗijĶ¬Ń§ĖłÅäÖʵÄĀČ»ÆÄĘČÜŅŗÖŠĪ¢Į£øöŹż±ČĪŖ1£ŗ18£»
“ĖŹ±ČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹżĪŖ£ŗ$\frac{36.5}{36.5+18”Į18}$”Į100%”Ö10.1%£»
£Ø5£©ČÜŅŗŹŌ¼ĮĘæ±źĒ©Ó¦³öŹ¾Ź²Ć“ČÜŅŗŗĶÅØ¶Č“óŠ”£»
£Ø6£©ČōÅäÖĘĖłµĆµÄĀČ»ÆÄĘČÜŅŗÖŠĀČ»ÆÄʵÄÖŹĮæ·ÖŹżĘ«Š”£¬æÉÄܵÄŌŅņŹĒĀČ»ÆÄĘÉŁĮĖ»ņĖ®¶ąĮĖ£»
A”¢³ĘĮæĀČ»ÆÄĘŹ±£¬ÓĪĀė²»ŌŚĮćĪ»ÖĆ¾Ķµ÷½ŚĢģĘ½Ę½ŗā£¬ŗó½«ÓĪĀėŅĘ¶ÆµĆµ½¶ĮŹż£¬“Ó¶ųŹ¹³ĘĮæµÄĀČ»ÆÄĘÉŁÓŚĖłŠčµÄĀČ»ÆÄĘ£¬ČÜÖŹÖŹĮæ·ÖŹżĘ«Š”£»
B”¢³ĘČ”Ź±±ź³ßÉĻµÄ¶ĮŹż”°3”±ŌŚÓĪĀėµÄÓŅ²ą£¬“Ó¶ųŹ¹³ĘĮæµÄĀČ»ÆÄĘÉŁÓŚĖłŠčµÄĀČ»ÆÄĘ£¬ČÜÖŹÖŹĮæ·ÖŹżĘ«Š”£»
C”¢ÓŠÉŁĮæ¹ĢĢåÉ¢ĀäŌŚĶŠÅĢ»ņĮōŌŚ³ĘĮæÖ½ÉĻ£¬»įµ¼ÖĀĀČ»ÆÄĘÖŹĮæĘ«Š”£¬“Ó¶ųµ¼ÖĀÅäÖʵÄĀČ»ÆÄĘČÜŅŗÖŠČÜÖŹÖŹĮæ·ÖŹżĘ«Š”£»
D”¢ĮæČ”Ė®Ź±ø©ŹÓ¶ĮŹż»įµ¼ÖĀĮæČ”µÄĖ®µÄÖŹĮæĘ«Š”£¬“Ó¶ųµ¼ÖĀÅäÖʵÄĀČ»ÆÄĘČÜŅŗÖŠČÜÖŹÖŹĮæ·ÖŹżĘ«“ó£»
E”¢ÅäÖĘČÜŅŗµÄÉÕ±ÓĆÉŁĮæÕōĮóĖ®ČóĻ“£¬“Ó¶ųµ¼ÖĀĖ®µÄÖŹĮæĘ«¶ą£¬ÅäÖʵÄĀČ»ÆÄĘČÜŅŗÖŠČÜÖŹÖŹĮæ·ÖŹżĘ«Š”£»
F”¢×°ĘæŹ±£¬ÓŠÉŁĮæČÜŅŗČ÷³ö£¬²»Ó°ĻģČÜÖŹÖŹĮæ·ÖŹż£®
“š°ø£ŗ
£Ø1£©3g£» 47ml£»
£Ø2£©B£»¼ĢŠųĢķ¼ÓĀČ»ÆÄĘ£¬Ö±µ½ĢģĘ½Ę½ŗā£»
£Ø3£©¼õÉŁĪó²ī£»
£Ø4£©²£Į§°ō£»10.1%£»
£Ø6£©ABCE£®
µćĘĄ ±¾ĢāÄѶȓó£¬×ŪŗĻŠŌĒ棬Ć÷Č·Ņ»¶ØČÜÖŹÖŹĮæ·ÖŹżČÜŅŗµÄÅäÖĘµÄ²½Öč”¢ĖłŠčŅĒĘ÷”¢×¢ŅāŹĀĻīµČŹĒÕżČ·½ā“š“ĖĄąĢāµÄ¹Ų¼ü£®


 Ó®ŌŚæĪĢĆĆūŹ¦æĪŹ±¼Ę»®ĻµĮŠ“š°ø
Ó®ŌŚæĪĢĆĆūŹ¦æĪŹ±¼Ę»®ĻµĮŠ“š°ø ĢģĢģĻņÉĻæĪŹ±Ķ¬²½ŃµĮ·ĻµĮŠ“š°ø
ĢģĢģĻņÉĻæĪŹ±Ķ¬²½ŃµĮ·ĻµĮŠ“š°ø Ńō¹āæĪĢĆĶ¬²½Į·Ļ°ĻµĮŠ“š°ø
Ńō¹āæĪĢĆĶ¬²½Į·Ļ°ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
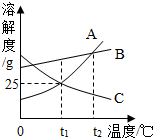 ČēĶ¼ŹĒA”¢B”¢CČżÖÖ¹ĢĢåĪļÖŹµÄČܽā¶ČĒśĻßĶ¼£¬øł¾ŻĒśĻßĶ¼»Ų“šĻĀĮŠĪŹĢā£®
ČēĶ¼ŹĒA”¢B”¢CČżÖÖ¹ĢĢåĪļÖŹµÄČܽā¶ČĒśĻßĶ¼£¬øł¾ŻĒśĻßĶ¼»Ų“šĻĀĮŠĪŹĢā£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
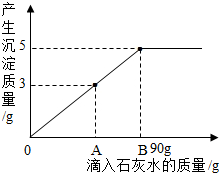 ŌŚŅ»ÉÕ±ÖŠŹ¢ÓŠNa2CO3 ŗĶNaOHµÄ»ģŗĻĪļ15g£¬ĻņĘäÖŠ¼ÓČė100gĖ®£¬¹ĢĢåČ«²æČܽā£®ĻņĖłµĆČÜŅŗÖŠµĪ¼Ó³ĪĒåŹÆ»ŅĖ®£¬²śÉś³ĮµķµÄÖŹĮæÓėµĪČėŹÆ»ŅĖ®µÄÖŹĮæ¹ŲĻµĒśĻßČēĶ¼ĖłŹ¾£®Ēėøł¾ŻĢāŅā»Ų“šĻĀĮŠĪŹĢā£ŗ
ŌŚŅ»ÉÕ±ÖŠŹ¢ÓŠNa2CO3 ŗĶNaOHµÄ»ģŗĻĪļ15g£¬ĻņĘäÖŠ¼ÓČė100gĖ®£¬¹ĢĢåČ«²æČܽā£®ĻņĖłµĆČÜŅŗÖŠµĪ¼Ó³ĪĒåŹÆ»ŅĖ®£¬²śÉś³ĮµķµÄÖŹĮæÓėµĪČėŹÆ»ŅĖ®µÄÖŹĮæ¹ŲĻµĒśĻßČēĶ¼ĖłŹ¾£®Ēėøł¾ŻĢāŅā»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ėü²»ÄÜÓėŹÆ»ŅĖ®·“Ó¦ | |
| B£® | ³¬ĮŁ½ē¶žŃõ»ÆĢ¼ŹĒŗĻ³ÉµÄŅ»ÖÖŠĀĪļÖŹ | |
| C£® | ³¬ĮŁ½ēCO2Į÷ĢåŠŌÖŹĢŲŹā£¬µ«ĖüÓėøɱłµÄ»Æѧ×é³ÉĻąĶ¬ | |
| D£® | ³¬ĮŁ½ēCO2Į÷ĢåŹĒŅ»ÖÖĢŲŹāµÄĪļÖŹ£¬ČŻŅ×Č¼ÉÕ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com