
| A£® | ¼×“¼ŗĶŅŅ“¼¶¼ÓÉĢ¼”¢Ēā”¢ŃõČżÖÖŌŖĖŲ×é³É | |
| B£® | ŅŅ“¼µÄĻą¶Ō·Ö×ÓÖŹĮæ±Č¼×“¼¶ą14 | |
| C£® | ¼×“¼ÖŠĢ¼ŌŖĖŲµÄÖŹĮæ·ÖŹż±ČŅŅ“¼ÖŠĢ¼ŌŖĖŲµÄÖŹĮæ·ÖŹż“ó | |
| D£® | ¼×“¼ŗĶŅŅ“¼³ä·ÖČ¼ÉÕ¶¼Éś³ÉCO2ŗĶH2O |
·ÖĪö A”¢øł¾Ż»ÆѧŹ½µÄŅāŅå½ųŠŠ·ÖĪöÅŠ¶Ļ£®
B”¢øł¾ŻĻą¶Ō·Ö×ÓÖŹĮæĪŖ¹¹³É·Ö×ÓµÄø÷Ō×ÓµÄĻą¶ŌŌ×ÓÖŹĮæÖ®ŗĶ£¬½ųŠŠ·ÖĪö½ā“š£®
C”¢øł¾ŻĪļÖŹÖŠŌŖĖŲµÄÖŹĮæ·ÖŹż¹«Ź½½ųŠŠ½ā“š£®
D”¢øł¾ŻÖŹĮæŹŲŗć¶ØĀɽųŠŠ·ÖĪö½ā“š£®
½ā“š ½ā£ŗA”¢¼×“¼ŗĶŅŅ“¼¶¼ÓÉĢ¼”¢Ēā”¢ŃõČżÖÖŌŖĖŲ×é³É£¬¹ŹĖµ·ØÕżČ·£®
B”¢ŅŅ“¼µÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ£ŗ12”Į2+1”Į6+16=46£»¼×“¼µÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ£ŗ12+4+16=32£¬Ņņ“ĖŅŅ“¼µÄĻą¶Ō·Ö×ÓÖŹĮæ±Č¼×“¼¶ą14£¬¹ŹĖµ·ØÕżČ·£®
C”¢¼×“¼ÖŠĢ¼ŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ$\frac{12}{32}$”Į100%=37.5%£¬ŅŅ“¼ÖŠĢ¼ŌŖĖŲµÄÖŹĮæ·ÖŹż=$\frac{24}{46}$”Į100%”Ö52.2%£¬Ņņ“Ė¼×“¼ÖŠĢ¼ŌŖĖŲµÄÖŹĮæ·ÖŹż±ČŅŅ“¼ÖŠĢ¼ŌŖĖŲµÄÖŹĮæ·ÖŹżŠ”£¬¹ŹĖµ·Ø“ķĪó£®
D”¢¼×“¼ŗĶŅŅ“¼¶¼ÓÉĢ¼”¢Ēā”¢ŃõČżÖÖŌŖĖŲ×é³É£¬øł¾ŻÖŹĮæŹŲŗć¶ØĀÉæÉÖŖ£¬¼×“¼ŗĶŅŅ“¼³ä·ÖČ¼ÉÕ¶¼Éś³É¶žŃõ»ÆĢ¼ŗĶĖ®£¬¹ŹĖµ·ØÕżČ·£®
¹ŹŃ”C£®
µćĘĄ ±¾ĢāÄŃ¶Č²»“ó£¬æ¼²éĶ¬Ń§ĆĒĮé»īŌĖÓĆ»ÆѧŹ½µÄÓŠ¹Ų¼ĘĖć½ųŠŠ·ÖĪöĪŹĢā”¢½ā¾öĪŹĢāµÄÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1.6g | B£® | 2.56g | C£® | 3.2g | D£® | 4.8g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ³Įµķ | B£® | ¹żĀĖ | C£® | Īüø½ | D£® | ¾²ÖĆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
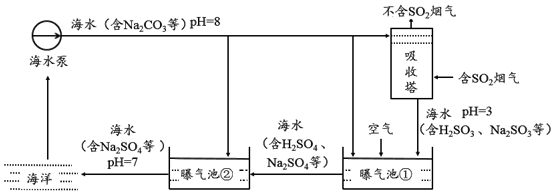
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
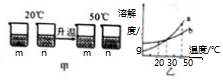
| A£® | Ķ¼ŅŅÖŠa±ķŹ¾mµÄČܽā¶ČĒśĻß | |
| B£® | Ķ¼¼×20”ęČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżm“óÓŚn | |
| C£® | Ķ¼¼×50”ꏱµÄm”¢nČÜŅŗŅ»¶ØŹĒ²»±„ŗĶČÜŅŗ | |
| D£® | 30”ꏱ£¬a”¢bČÜŅŗČÜÖŹÖŹĮæ·ÖŹż²»ĻąµČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĪŖĮĖ²āĮæŃĄøąµÄpH£¬æÉŅŌ½«ŃĄøą¼·µ½²£Į§±ÖŠ£¬¼ÓĖ®½Į°čŗóÓĆpHŹŌÖ½Ą“²āĮæ | |
| B£® | ²»ŠāøÖĖ®ŗųÓĆ¾ĆŗóŅײśÉśĖ®¹ø£¬æÉŅŌÓĆ“×Ėį³¤Ź±¼ä½žÅŻµÄ·½Ź½Ą“³żĖ®¹ø | |
| C£® | ×ŌĄ“Ė®³§Ķعż¼ÓŠõÄż¼ĮĪüø½”¢¹żĀĖ”¢»īŠŌĢæĪüø½”¢Ķ¶Ņ©Ļū¶¾µČĮ÷³Ģæɽ«×ŌČ»½ēµÄĖ®¾»»Æ | |
| D£® | ĪŖĮĖÖĪĮĘĪøĖį¹ż¶ą£¬æÉŅŌ×ńŅ½Öö·žÓĆijŠ©ŗ¬ÓŠ¼īŠŌµÄĪļÖŹ£¬Čē£ŗĒāŃõ»ÆÄĘ”¢Š”ĖÕ“ņ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  ĒāČ¼ĮĻĘū³µ | B£® |  ·ēĮ¦Ė®³µ | C£® |  ŗĖ¶ÆĮ¦Ē±Ķ§ | D£® |  Ģ«ŃōÄÜĀ·µĘ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com