
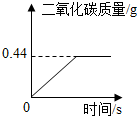
 �ڡ���ʽ�����Ƽ���Ĺ��������У����һ�����ü���̼�����Ƶķ�������ȡ���ij�����������ƵõIJ�ƷNa2CO3�л�������NaHCO3��Ϊ�˲ⶨ��Ʒ��Na2CO3������������ȡ200g�û������ȣ���Ӧ���������ɶ�����̼����������뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ������ʾ��2NaHCO3
�ڡ���ʽ�����Ƽ���Ĺ��������У����һ�����ü���̼�����Ƶķ�������ȡ���ij�����������ƵõIJ�ƷNa2CO3�л�������NaHCO3��Ϊ�˲ⶨ��Ʒ��Na2CO3������������ȡ200g�û������ȣ���Ӧ���������ɶ�����̼����������뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ������ʾ��2NaHCO3
| ||
| ||
| 168 |
| x |
| 44 |
| 0.44g |
| 200g-1.68g |
| 200g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ϡ��ϳ����ϳ���ά���Ͻ����л��ϳɲ��� |
| B��ʯī��C60���������ǵ��ʣ����ʯ������ʯ��ú��ʯ���ǻ���� |
| C������Ӫ������ָ�����ʡ����ࡢ��֬��ά���ء����Ρ�ˮ |
| D������ȱп�ͻ������״���״� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ǧ���������ɽ--�Ӵ�ɽ�↑��ʯͷ�����������仯 |
| B���һ����������--ʯ��ʯ�ڸ����·����˻�ѧ�仯 |
| C����ë����ˮ--����ˮ�ϵĶ��ܵ��ĸ����������� |
| D�����Ʋ��岨--������������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ľ̿��������ȼ�ղ���������̼ |
| B����˿�������о���ȼ�գ��������� |
| C������ڿ�����ȼ�շ���ҫ�۵İ� |
| D�������ڿ�����ȼ�շ�������ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com