
| ʵ����� | ʵ������ | ʵ����� |
| | | |
| ʵ����� | ʵ������ | ʵ����� |
| ȡ������Ʒ���Թ��У���ˮ���ã����ϲ���Һ�е����̪��Һ��ȡ�²㲻����μ����� | �²��в�����ϲ���壬��Һ��������ݲ��� | ���ʵĸ�����ɷ�Ϊ�������ƺ�̼��� |
| ʵ����� | ʵ������ | ʵ����� |
| ȡ������Ʒ���Թ��У���ˮ���ã����ϲ���Һ�е����̪��Һ��ȡ�²㲻����μ����� | �²��в�����ϲ���壬��Һ��죬�����ݲ��� | ���ʵĸ�����ɷ�Ϊ�������ƺ�̼��� |
| ʵ����� | ʵ������ | ʵ����� |
| ȡ������Ʒ���Թ��У���ˮ���ã����ϲ���Һ�е����̪��Һ��ȡ�²㲻����μ����� | �²��в�����ϲ���壬��Һ����ɫ�������ݲ��� | ���ʵĸ�����ɷ�Ϊ̼��� |


 Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д� �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
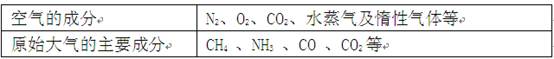
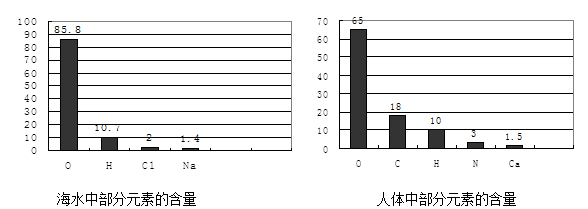
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
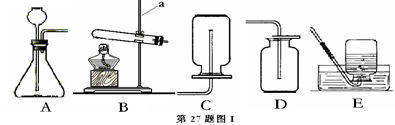
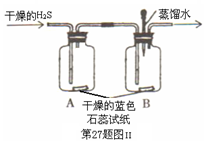
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

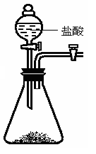
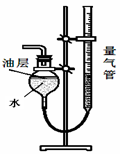
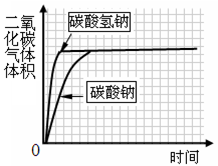
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 Cu+H20��CO+CuO
Cu+H20��CO+CuO Cu+CO2������Ӧ��H2��CO�����ܽ�CuO��ԭΪCu�����ʣ��ڷ�Ӧ������ԭ����
Cu+CO2������Ӧ��H2��CO�����ܽ�CuO��ԭΪCu�����ʣ��ڷ�Ӧ������ԭ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
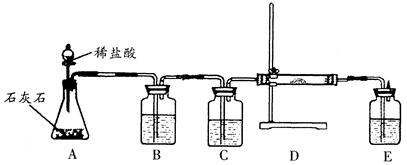
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
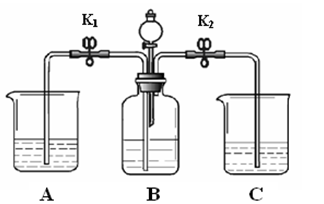
| λ�� | A | B | C | ��Һ©�� |
| ҩƷ | ����ʯ��ˮ | ̼������Һ | ����ʯ��ˮ | ϡ���� |
| ���� | �ر�K1����K2�ͷ�Һ©���Ļ����������μ�ϡ���� | |||
| ����д�� C�з�Ӧ�Ļ�ѧ����ʽ | | |||
| ���� | �ر�K2����K1 | |||
| ���� | B��Һ���ص�������A�У�A���а�ɫ�������� | |||
| �������������ԭ�� | | |||
| λ�� | A | B | C | ��Һ©�� |
| ҩƷ | X��Һ | ϡ���� | ˮ������ʯ����Һ�� | ̼������Һ |
| ���� | �ر�K1����K2�ͷ�Һ©���Ļ����������μ�̼������Һ | |||
| ���� | | |||
| ���� | �ر�K2����K1 | |||
| ���� | B��Һ���ص�������A�У� A������ɫ������� | |||
| д��A��X��Һ������ | ������д���֣� | |||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڢۢ� | B���ڢ� | C���ۢ� | D���٢ۢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com