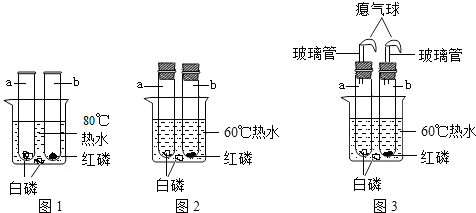
ʵ���ǻ�ѧѧ�ƽ��п�ѧ̽������Ҫ;�����������ػ�Ӱ�컯ѧ��Ӧ�Ľ��У�
��1������������������Ӧ�������������ף������������������������ף����о���ѧ����ͬʵ��
��
��2��31g����������Ӧ����������������������
��������������Ӧ����������������������
��
��3����31g����30g������Ӧʱ������Ϊ
��
��4��CO
2��NaOH�ķ�Ӧ��������Ӧ���ƣ�CO
2����ʱ��Ӧ���£�CO
2+NaOH�TNaHCO
3CO
2������ʱ��Ӧ���£�CO
2+2NaOH�TNa
2CO
3+H
2O����ͨ��CO
2ʱ��Ӧ���̿ɿ������½��У��ȷ����˷�ӦCO
2+2NaOH�TNa
2CO
3+H
2O��
��CO
2��ʣ�࣬��������Na
2CO
3+H
2O+CO
2�T2NaHCO
3������80g��������Ϊ10%��NaOH��Һ��������ͨ��6.6gCO
2�����������Na
2CO
3��NaHCO
3��������




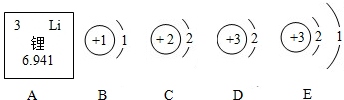
 ��ͼ�ش�
��ͼ�ش�