
 �����������ij���Ԫ�أ�ÿ�ձ������������ĸƣ�Ŀǰ�г��ϵIJ���ҩ���кܶ࣬��ͼ��ij��Ʒ�ƵIJ���ҩƷ�IJ���˵���飮
�����������ij���Ԫ�أ�ÿ�ձ������������ĸƣ�Ŀǰ�г��ϵIJ���ҩ���кܶ࣬��ͼ��ij��Ʒ�ƵIJ���ҩƷ�IJ���˵���飮���� ��1�����ݻ�������Ԫ�ص���������=$\frac{���ԭ��������ԭ�Ӹ���}{��Է�������}$��100%�����з������
��2�����ݸ�Ƭ�и�Ԫ�ص���������=$\frac{��Ƭ�и�Ԫ�ص�����}{��Ƭ������}$��100%�����з������
��3���������⣬ÿ�η���1Ƭ��ÿ��3�Σ�ÿƬ��Ƭ������Ϊ0.5g�������ÿ����ø�Ƭ�����������������ÿ�������Ԫ�ص�������
��� �⣺��1��CaCO3�и�Ԫ�ص���������Ϊ$\frac{40}{40+12+16��3}$��100%=40%��
��2��ÿƬ��CaCO3������Ϊ0.625g������Ԫ�ص�����Ϊ��0.625g��40%=0.25g��
ÿƬ��Ƭ������Ϊ1g�����Ƭ�и�Ԫ�ص���������Ϊ$\frac{0.25g}{1g}��$100%=25%��
��3���������⣬ÿ�η���1Ƭ��ÿ��2�Σ�ÿƬ��CaCO3������Ϊ0.625g������Ԫ�ص�����Ϊ��0.625g��40%=0.25g����ÿ�������Ԫ�ص�����Ϊ0.25g��2=0.5g��
�ʴ�Ϊ����1��40%����2��0.25��25%����3��0.5��
���� �����ѶȲ�����ͬѧ�ǽ�ϱ�ǩ����Ϣ��������û�ѧʽ���йؼ�����з������⡢��������������


 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
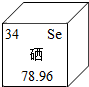 ��������������Ԫ�أ���Ԫ�����ڱ�����Ԫ�ص�ijЩ��Ϣ��ͼ��ʾ�������й�����˵����ȷ���ǣ�������
��������������Ԫ�أ���Ԫ�����ڱ�����Ԫ�ص�ijЩ��Ϣ��ͼ��ʾ�������й�����˵����ȷ���ǣ�������| A�� | ���ڽ���Ԫ�� | B�� | ԭ�Ӻ���������Ϊ34 | ||
| C�� | ԭ������Ϊ34 | D�� | ���ԭ��������78.96g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
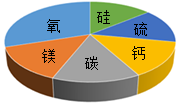 �����ij��ʯ����������Ԫ�ص�����������ͼ��ʾ����ÿ�ʯ�в����ܴ��ڵ������ǣ�������
�����ij��ʯ����������Ԫ�ص�����������ͼ��ʾ����ÿ�ʯ�в����ܴ��ڵ������ǣ�������| A�� | CaSO4 | B�� | SiO2 | C�� | MgCO3 | D�� | Fe2O3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
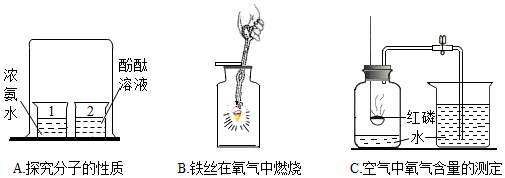
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ˮ�dz����������� | |
| B�� | ������������ˮ�����彡������ | |
| C�� | ������ķ������Խ���ˮ���� | |
| D�� | ˮ�����ȾԴ��Ҫ�ǹ�ũҵ��ˮ��������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  �μ�Һ�� | B�� |  �����Թ� | ||
| C�� |  ���Ӿƾ� | D�� |  ����Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
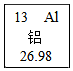 Ԫ�����ڱ���ѧϰ���о���ѧ�Ĺ��ߣ���ͼ����Ԫ�ص������Ϣ������˵���в���ȷ���ǣ�������
Ԫ�����ڱ���ѧϰ���о���ѧ�Ĺ��ߣ���ͼ����Ԫ�ص������Ϣ������˵���в���ȷ���ǣ�������| A�� | ���ǽ���Ԫ�� | B�� | ������������13 | ||
| C�� | ������������13 | D�� | �������ԭ��������26.98 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ԡ¶ϴȥ���ϵ���֬ | |
| B�� | �þƾ���ȥ�������Թ��ڱڵĵ� | |
| C�� | �����ͳ�ȥ�·��ϵ����� | |
| D�� | ����ˮϴȥ�������ձ��ײ�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com