
| A�����ơ�п��ͭ������������Ԫ�� |
| B������������л������ɵĻ���� |
| C�����DZ����зۣ�θ�����߷��ã���ֹʹ������θ������Ч�� |
| D�����Լӹ�������ơ���������ƵȲ�Ʒ�����Ϊ�� |




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
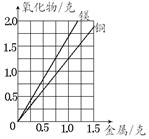
| A��þ��ͭ���ܶȲ�ͬ |
| B�����������þ��ͭ���ϵ���Ԫ��������ͬ |
| C�����Ȳ���֣�û����ȫ���� |
| D��ʵ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
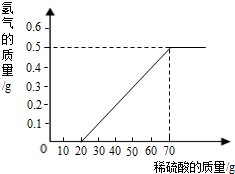
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| �������������g�� | 25.0 |
| ������+ʳ����Һ��g�� | 45.0 |
| ������+ʳ�ξ��壨g�� | 27.4 |
| ���ݴ��� | ��Һ��������������Ϊ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
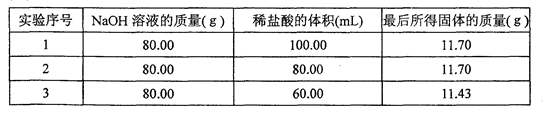
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| ���� | ��1�� | ��2�� | ��3�� |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 30 | a | 20 |
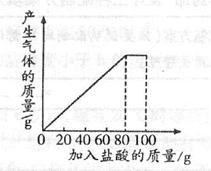
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com