
| ϡ��������� | ʣ���������� |
| ��һ�μ���10g | 3.0g |
| �ڶ��μ���10g | 2.0g |
| �������10g | 1.0g |
| ���Ĵμ���10g | 0.4g |
| 4g-0.4g |
| 4g |
| 100 |
| 1g |
| 73 |
| X |
| 0.73g |
| 10g |
| ||
| 100 |
| 90%y |
| 56 |
| 100t-10%y |


 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
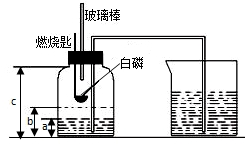 ijУ��ѧ�о���ѧϰС����ѧϰ�ˡ����������������ⶨ���Ļ����ϣ��Ľ��˽̲��е�ʵ�飬��Ƴ�����ͼ��ʾ��ʵ��װ�ã�ʵ�鲽�����£�
ijУ��ѧ�о���ѧϰС����ѧϰ�ˡ����������������ⶨ���Ļ����ϣ��Ľ��˽̲��е�ʵ�飬��Ƴ�����ͼ��ʾ��ʵ��װ�ã�ʵ�鲽�����£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��������Ĵ��� | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| �ձ�����������������/g | 78.9 | 97.8 | 116.7 | 135.60 | 155.05 | 175.05 | 195.05 |
| �������������/g | 1.1 | 2.2 | a | 4.4 | 4.95 | b | -- |
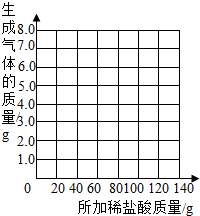
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
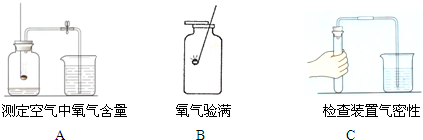
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ��ʵ�鷽�� | �� �� | CO��CuO��Ӧ�Ļ�ѧ����ʽ | ||||||||
Cu2O Cu2O |
ȡ������ɫ��ĩ�����Թ��У������м�������ϡ���ᣬ�� ȡ������ɫ��ĩ�����Թ��У������м�������ϡ���ᣬ�� |
��Һ��Ϊ��ɫ�����к�ɫ���� ��Һ��Ϊ��ɫ�����к�ɫ���� |
2CuO+CO
2CuO+CO
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ��Ŀ�� | ʵ����� | ���� | ���ۻ�ѧ����ʽ |
| ��ȥ̼���� | ȡ�����ù�����Ʒ����ˮ�����Һ���μ��������Ȼ�����Һ����ַ�Ӧ����� | ������ɫ���� ������ɫ���� |
�йط�Ӧ�Ļ�ѧ����ʽΪ Na2CO3+CaCl2=2NaCl+CaCO3�� Na2CO3+CaCl2=2NaCl+CaCO3�� |
| �����Ƿ����������� | ����˺����Һ�е��� ��̪��Һ ��̪��Һ |
��� ��� |
����Ʒ�к����������� |
| ��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� | ����� | ������ | ���ߴ� |
| ��ƿ����ʢ���ʵ������� | 95.00 | 120 | 145 | 170 | 192.8 | 215.6 | 240.6 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com