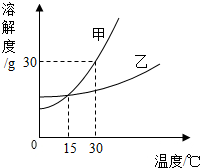
27��ʵ��������һƿ��ǩ��ȱ�����ᣬ������������й���������֣�
��1����������ȷ����ƿ������Ũ������˲²��ǣ����˼ǵú����ǣ���Ҹ����Ѽ�������Ϊ��Ӧ���������е�
C
�����������ţ�
A������ B������ C��ʵ�� D������
����������
��ƿ�ǣ��۲�ƿ���Ϸ��Ƿ��а�������
��
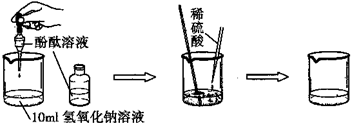
��2��ijͬѧ����Բⶨ������ÿ�����к�����HCl����������������ͼ��кͷ�Ӧ��ԭ���������һ��ʵ�鷽����������������£�
��һ������С�ձ��м���10g5%��NaOH��Һ�� �ڶ������������ձ��е���2�η�̪��Һ��
����������10mL����Ͳ��ע���������һ���̶ȣ�
���IJ����ý�ͷ�ι���ȡ��Ͳ�е����ᣬ��ε��������ձ��У��ε���Һ�ɺ�ɫ�ոձ�Ϊ��ɫΪֹ������ͷ�ι��е�ʣ��Һ��ȫ��������Ͳ�У�
���岽����¼ʵ�����ݣ�
������������ȷ��ÿ���������к�����HCl��������
���������ʵ����̣��ش��������⣺
�ٱ�ʵ��ΪʲôҪ��ָʾ������̪������
��ָʾ������ȷ������μӵ�ʲôʱ����������������ǡ����ȫ��Ӧ��
��
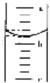
�ڵ�����������Ͳע�����ᣬ��Ͳ��Һ���λ����ͼ��ʾ��a��b��b��c�̶ȼ�����1mL������̶�aΪ4����Ͳ������������
3.2ml
��
�۵��IJ�Ҫ�õ���������������;��
����
��Ŀ����
��������������Ƴ�ֽӴ���ʹ�䷴Ӧ��ȫ��
��
�ܵ��岽Ҫ��¼��������
��Ͳ��ʣ���������
��
��3��ʵ��������е�ͬѧ��Ϊ���ڣ�2���вⶨÿ���������к�����HCl��������ԭ���ͷ������ж��֣�����������ѧ�Ļ�ѧ֪ʶ��д���������������������ּ��������ݵ�ԭ����Ҫ�ⶨ�����ݣ�����Ҫд��ѧ����ʽ����
����һ��
����һ��С�ձ�������������ձ��м���һ���������ᣬ�ټ���һ�����Ĺ�����п������������ð������װ�õ�����������
Ӧǰ��װ�ü��ٵ�����Ϊ��������������������������������������������ʵ�����
��
��������
���ձ��м���һ�����ĵ����ᣬ�ټ���һ�����Ĺ���������ͭ������ַ�Ӧ���ˡ�ϴ�ӡ����ﲢ����ʣ������ͭ����������
�ݲμӷ�Ӧ������ͭ������������������������ʵ�����
��
��������
����һ��С�ձ�������������ձ��м���һ���������ᣬ�ټ���һ�����Ĺ����Ĵ���ʯ����������ð������װ�õ���������
��Ӧǰ��װ�ü��ٵ�����Ϊ������̼�����������ݶ�����̼������������������������ʵ�����
��



 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�

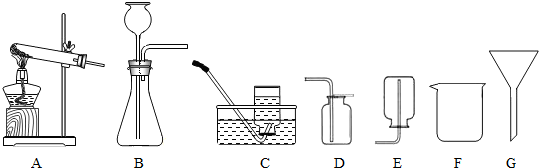
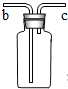
 ��2006?�Ͳ���������ͬѧ����ѧϰ��Һ֪ʶʱʵ�鱨���еIJ������ݣ���������ش�
��2006?�Ͳ���������ͬѧ����ѧϰ��Һ֪ʶʱʵ�鱨���еIJ������ݣ���������ش�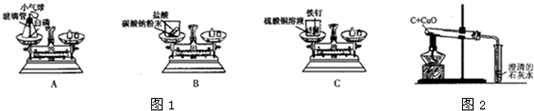
 ������ͬѧ����ѧϰ��Һ֪ʶʱʵ�鱨���еIJ������ݣ���������ش�
������ͬѧ����ѧϰ��Һ֪ʶʱʵ�鱨���еIJ������ݣ���������ش�