
ij̼������Ʒ�к��������Ȼ������ʣ�Ϊ�ⶨ����Ʒ��̼���Ƶ���������������������ʵ�飺 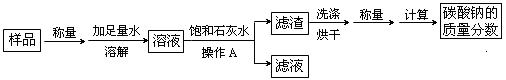
��ش��������⣺
��1������A��������________________���ò����������õ��IJ����������ձ�������� ��
��2����ʵ������м��뱥��ʯ��ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
��3��Ϊ̽��������Ӧ����Һ�е����ʳɷ֣���ͬѧ����Һ�еμӹ���ϡ���ᣬ���������ݲ�������μ�����ǰ��Һ�е����ʳ��Ȼ������ ���μ�����Ĺ����з�����Ӧ�Ļ�ѧ����ʽΪ ____________��
��1������ ©���������� ��2��Ca(OH)2+Na2CO3=CaCO3��+2NaOH
��3�� Na2CO3��4��Na2CO3+2HCl=2NaCl+H2O+CO2��
���������������1�����ˣ����ڷ��������Թ�����Һ�壨������Թ��壩�����Բ���A�������ǹ��ˣ��ò����������õ��IJ����������ձ�������У�©����������
��2��̼�����к����Ȼ������ʣ����뱥��ʯ��ˮ��ֻ��̼��������ʯ��ˮ��Ӧ����ѧ����ʽ�ǣ�Ca(OH)2+Na2CO3=CaCO3��+2NaOH
��3����ͬѧ����Һ�еμӹ���ϡ���ᣬ���������ݲ�����˵����Һ�к���̼���μ� Na2CO3��������Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl=2NaCl+H2O+CO2��
���㣺���˲�����̼���ε�����


 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ϸ�۲�������Һ�ı仯���ش����⡣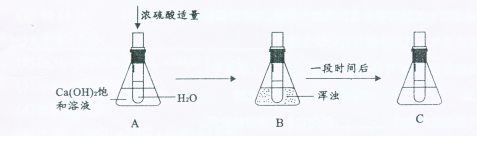
��1��Ũ�������ɫ�� ��
��2��B����Һ����ǵ�ԭ�� ��
��3����Ũ���ỻ���������ƹ��� ����ǡ�������ͼB����ͬ����
��4��ϡ�������Ȼ�����Ӧ�ķ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����H��O��C��Cl��Na��Ca����Ԫ����ѡ���ʵ�Ԫ�أ���ɷ�������Ҫ������ʣ������л�ѧʽ����ո��С�
���˹������������ ��
�ڿ����ڽ������������� ��
�ۿ��������ͷۺ�����θ�����֢���� ��
�ܿ����������ϵ��� ��
��2���˵�θҺ�к������������ᡣ��Ҫ���йصĻ�ѧ����ʽ��д�ں����ϡ�
��θ�����ʱ�������ú�������������ҩ���������___________________________��
��Ba2+�ж�����X�����θ��ʱ�����ñ��ͣ����ᱵ���������̼�ᱵ�������ж������е�ԭ��Ϊ ��
�����̼�ᱵ�ж������������кҩ������þ�����ⶾ��ԭ���� ��
��3���ܽ�ͬѧ���˸������ͣ�����һ�����ʡ���Ƥ������ӡ����ͼ��ʾ�̱꣬���ᵽһ�ɴ̼�����ζ���ܽ���˸�����˵��
����������ڻ����е� ������ţ���
A.�ط� B.���� C.��
ÿ���û����к���Ԫ�ص���������Ϊ kg��
�������Ӧ���е������� ������ţ���
A.������ˮ B.�лӷ��� C.�����ֽ�
�����������ǿ�Ӧ���������ռӦ�ķ�Ӧ����ʽΪ��
NH4NO3 + NaOH �� NaNO3 + NH3�� + X����X�Ļ�ѧʽΪ ��ʩ�ø������ʱ��Ҫ������ ��ѡ��ᡱ��������ʻ��ã�����ή�ͷ�Ч��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ�����������ߣ��������ǵ�����ϢϢ��ء���ʯ��ʯ��̼�����ơ�ϡ���ᡢʳ������������ѡ����������;���Ӧ�����ʣ���д�ڿհ״���
(1)�����ڱ��Ƹ��ʱ�ķ��ͷ�________________________��
(2)���ʱ����ζƷ����______________________________��
(3)�������������ϵ���______________________________��
(4)�����ڳ�ȥ������������������__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʢ�����ǣ�����Ҫ�ɷ�ΪCaCO3���Ա��Ǻʹ���Ϊԭ�ϣ������ռ�ļ�Ҫ�����������£�
��ش��������⣺
��1��д���ٷ�����Ӧ�Ļ�ѧ����ʽ ��
��2��д���۷�����Ӧ�Ļ�ѧ����ʽ ��
��3���ڹ�ҵ�ϣ���ʯ�ҿ����������ռ��ũҵ�ϣ�����һ����;�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������������Ӫ��Һ���������һ�ַ�����
��1��ijͬѧҪ��ʵ��������150g������������Ϊ2%���������Һ����Ҫ����ص�����
Ϊ g��
��2������һ����ɫ��Ӫ��Һ��������KNO3��Ca��NO3��2��K2CO3��KCl�е�һ�ֻ���������ɣ�Ϊ̽����ɷ֣�ijͬѧ��Ʋ��������ͼ��ʾ��ʵ�飮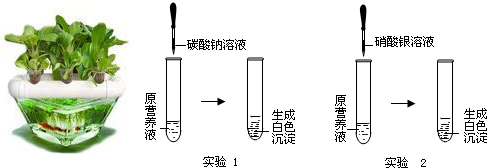
��������ʵ�飬��������й��ƶϣ�
����ʵ��1��ȷ��ԭӪ��Һ��һ��û�е������� ��
�ڸ���ʵ��1��ʵ��2�Ʋ�ԭӪ��Һ����ɿ����� �������
�������ԭӪ��Һ��K+��Clһ����Ŀ֮��Ϊ1��1����ԭӪ��Һ������е�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ������������Һ�������õμӷ�ʽ��Ӧʱ����ҺpH�������Һ����仯�����ߡ�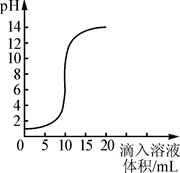
��1���������ƺ�����ǡ����ȫ��Ӧʱ����Һ��pH 7������ڡ���С�ڡ����ڡ�����
��2�����������жϣ��÷�Ӧ�ǽ� �������������Һ�������ᡱ����ͬ������ �У������� ��
��3����������Һ�����Ϊ5mLʱ��������Һ�е�����Ϊ ��д��ѧʽ�������ڴ���Һ�е���ʯ����Һ����Һ�� ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ǻ�ѧʵ�����г��õ��Լ���
��1���������ƹ�����¶�ڿ����У���������ˮ�ֶ�ʹ���渲��һ����Һ����һ�����ֽС����⡱������������ ������һ�仯�Ļ�ѧ����ʽ�ɱ�ʾΪ�� �������������ƹ�������ܷⱣ�森
��2��ʵ����ʢ������������Һ���Լ�ƿ�����ò���������ԭ�����ڳ����£����������벣���еĶ������軺���ط�����Ӧ������ʹƿ����ƿ��ճ����һ�𣬷�Ӧ�Ļ�ѧ����ʽΪ��SiO2+2NaOH�TX+H2O�����ƶ�X�Ļ�ѧʽΪ�� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ������������������ء������������ʵ������ա�
�ٵ�������̼�����ƣ��۶�����̼����ľ̿�����Ȼ��ơ�
��1���������Ƹ��ķ��ͷ۵���Ҫ�ɷ��� ��
��2����������У���������ζ���� ��
��3������������������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com